Session Information
Session Type: Poster Session C
Session Time: 9:00AM-11:00AM
Background/Purpose: DARWIN 3 (NCT02065700) is a long-term extension (LTE) study assessing the safety and efficacy of filgotinib (FIL) in patients with rheumatoid arthritis (RA) and an inadequate response to methotrexate (MTX).1 In the DARWIN 1 (NCT01888874) and DARWIN 2 (NCT01894516) parent studies, patients received FIL in combination with MTX or FIL monotherapy, respectively. This analysis aimed to provide an update on the safety and efficacy of FIL 200 mg (FIL200) in patients with RA, with or without MTX, with a maximum of 8.2 years of exposure.
Methods: Patients completing the DARWIN 1 (FIL + MTX) and DARWIN 2 (FIL monotherapy) Phase 2 studies could enter DARWIN 3, receiving FIL200. The proportion of patients experiencing treatment-emergent adverse events (TEAEs) were reported using the safety analysis set, comprising data from both the parent and LTE studies. Efficacy was assessed from LTE baseline using the American College of Rheumatology (ACR) 20/50/70 improvement criteria and Disease Activity Score in 28 joints using C-reactive protein (DAS28-CRP), up to 264 weeks. Low disease activity and remission were defined as DAS28-CRP ≤ 3.2 and < 2.6, respectively.
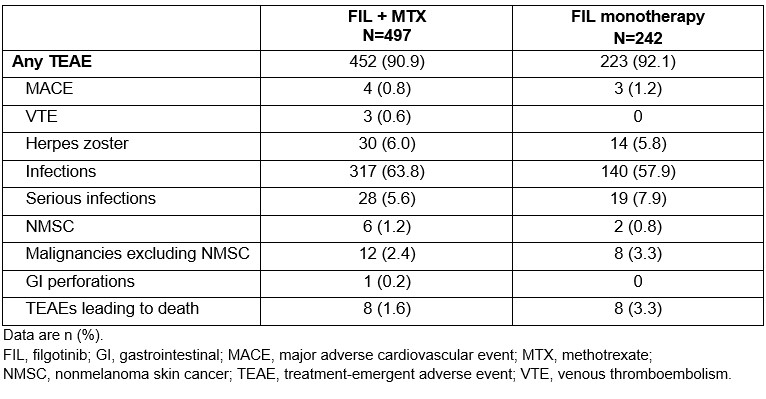
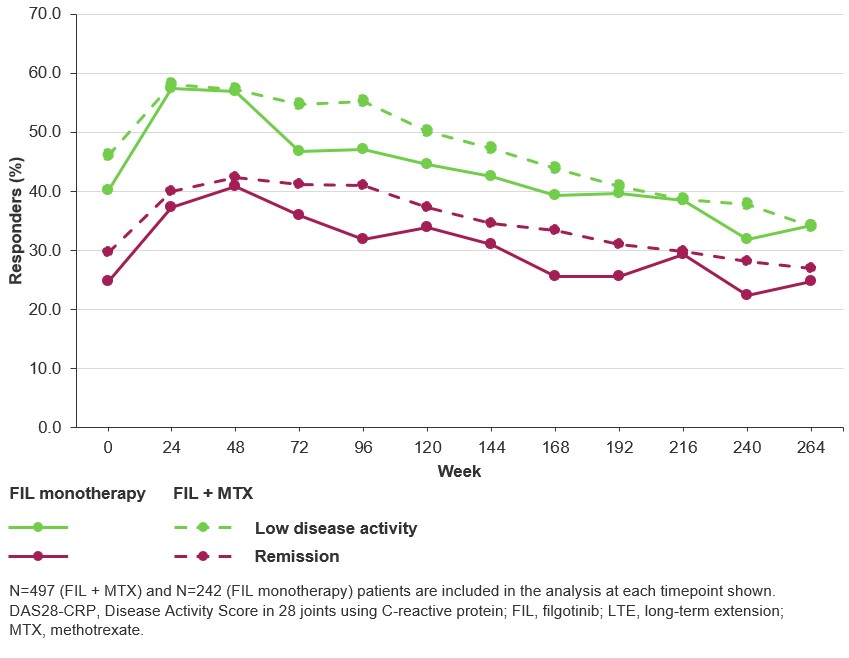
Results: In total, 739 patients were enrolled in DARWIN 3. Mean (standard deviation; SD) FIL exposure was 4.89 (2.72) years in the FIL + MTX group and 4.78 (2.79) years in the FIL monotherapy group. In the FIL + MTX vs FIL monotherapy groups, TEAEs were reported for 90.9% and 92.1% of patients, respectively (Table). The most common TEAE was infection. In both treatment groups, 8 patients had a TEAE leading to death (1.6% and 3.3%, respectively). Exposure-adjusted incidence rates, censored at time of first event for major adverse cardiovascular event, venous thromboembolism, herpes zoster, infections, serious infections, nonmelanoma skin cancer (NMSC), malignancies excluding NMSC, gastrointestinal perforations and TEAEs leading to death, will be reported. Through 5 years, ACR20/50/70 responses were maintained in 86.3%/66.7%/50.7% of the FIL + MTX group and 90.8%/74.8%/51.4% of the FIL monotherapy group, respectively (observed data). DAS28-CRP low disease activity and remission rates (nonresponder imputation) at DARWIN 3 baseline were 46.1%/40.1% (FIL + MTX) and 29.6%/24.8% (FIL monotherapy) (Figure). At Week 264, the proportion of patients achieving low disease activity and remission were 34.0%/34.3% (FIL + MTX) and 27.0%/24.8% (FIL monotherapy).
Conclusion: With a maximum of 8.2 years of exposure in patients with RA, the FIL safety profile is similar between the background MTX and monotherapy treatment arms. Both arms show sustained efficacy over time.
Reference:
1. Kavanaugh A, et al. J Rheumatol 2021;48:1230–8
To cite this abstract in AMA style:
Westhovens R, Alten R, Dagna L, Kavanaugh A, Withrop K, Barry J, Besuyen R, Corallo C, de Vries D, Martin N, Watson C, Genovese M, Spindler A, Stanislavchuk M, Greenwald M, Emery P. Safety and Efficacy of Filgotinib: An Update from the DARWIN 3 Phase 2 Long-term Extension with a Maximum of 8.2 Years of Exposure [abstract]. Arthritis Rheumatol. 2023; 75 (suppl 9). https://acrabstracts.org/abstract/safety-and-efficacy-of-filgotinib-an-update-from-the-darwin-3-phase-2-long-term-extension-with-a-maximum-of-8-2-years-of-exposure/. Accessed .« Back to ACR Convergence 2023
ACR Meeting Abstracts - https://acrabstracts.org/abstract/safety-and-efficacy-of-filgotinib-an-update-from-the-darwin-3-phase-2-long-term-extension-with-a-maximum-of-8-2-years-of-exposure/