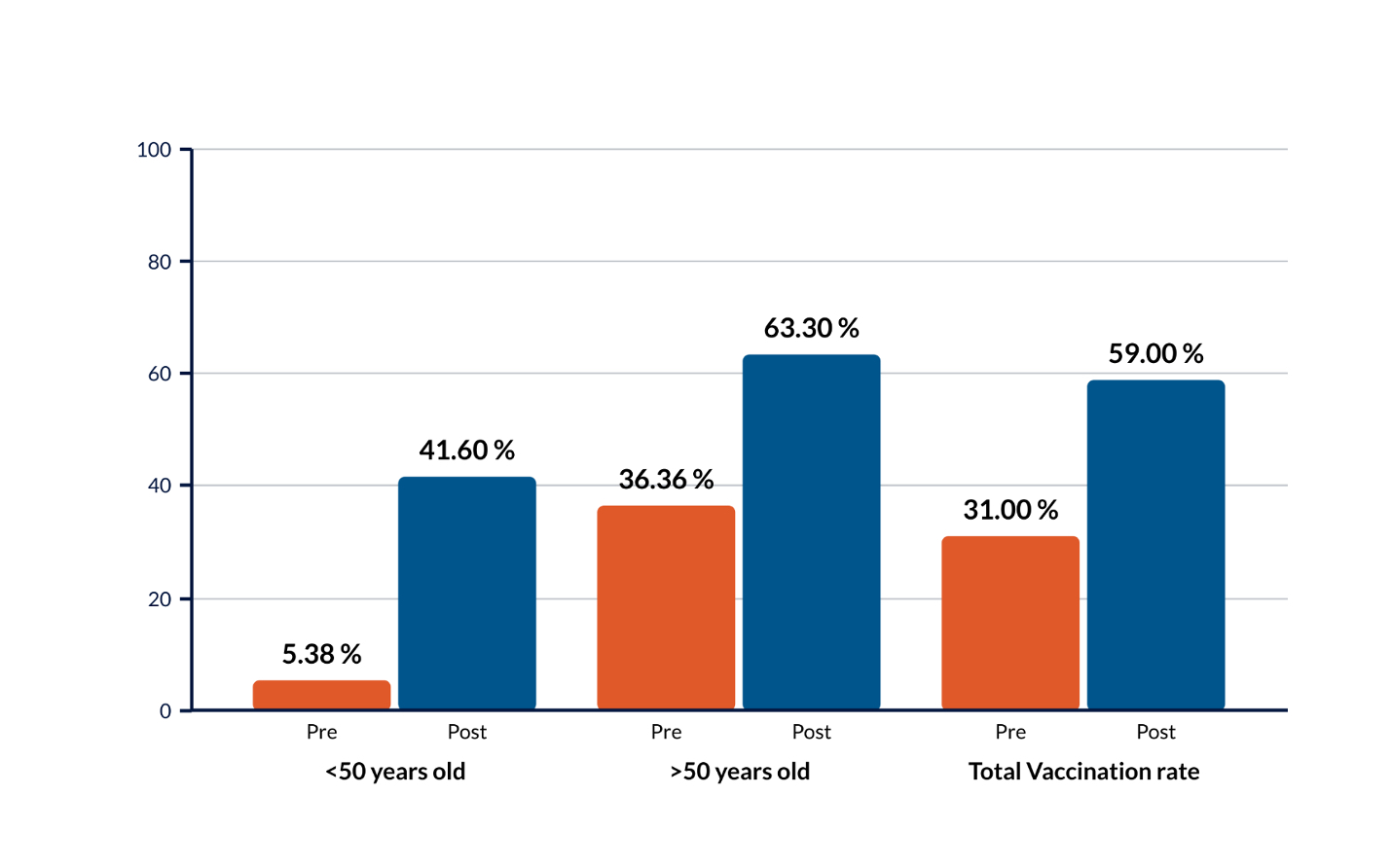Session Information
Session Type: Poster Session C
Session Time: 9:00AM-11:00AM
Background/Purpose: Herpes zoster is a painful rash that involves one to three adjacent dermatomes, secondary to reactivation of latent varicella-zoster virus (VZV). About 95 % of the U.S population has been exposed to VZV, and therefore are at risk of developing herpes zoster. Many patients with rheumatologic conditions are at higher risk for herpes zoster and related complications.
Since 2017, The Centers for Disease Control and Prevention has recommended the 2-dose series recombinant zoster vaccination (RZV) in all immunocompetent adults aged ≥50 years. In 2021, this recommendation was expanded to include adults aged ≥18 years who are or will be immunodeficient or immunosuppressed because of disease or therapy. This recommendation includes patients with rheumatologic conditions or patients on immunosuppressive medications. This Quality Improvement project was designed to increase the RZV vaccination rate to 50% in patients receiving immunosuppressive therapy in the rheumatology clinic at the Orlando VA Healthcare System.
Methods: We identified patients aged ≥18 years who were prescribed biologics, Janus Kinase (JAK) inhibitors and/or conventional synthetic disease modifying antirrheumatic drugs (csDMARDs), from 2/2021 until 2/2022, who had not received RZV vaccination. We conducted a root cause analysis to identify barriers for RZV vaccination. Our interventions included a visual aid in patient rooms encouraging patients to ask about RZV vaccination, pre-visit screening for RZV vaccination done by nurses and providers, set up a process on how to order RZV vaccine in satellite clinics, presentation to rheumatology faculty about baseline metrics, and providing education on the RZV vaccine at each clinic visit. We then obtained follow-up data from 5/2022 until 4/2023.
Results: A total of 808 patients aged ≥18 years who were prescribed immunosuppressive medications in the rheumatology clinics from 2/2021 until 2/2022 were identified. Of these patients, 67% (n=540) had not received RZV vaccination, and 418 of these patients were ≥50 years of age. Therefore, only 33% of our patients on immunosuppressive therapy have been appropriately vaccinated against VZV.
After our intervention, we identified 761 patients aged ≥18 years who were prescribed biologics or JAK inhibitors from 5/2022 until 4/2023. Of these patients, 41% (n=309) had not received RZV vaccination and 229 of these patients were ≥50 years of age. Therefore, RZV vaccination rate increased from 33% to 59% (Figure 1).
There is a numerically increase of the percentage of immunosuppressed patients who have been appropriately vaccinated against VZV. The total of patients in the pre-intervention group is higher than post-intervention, and this could be related to changes in medication regimen or geographic relocation.
Conclusion: Results from our quality improvement project showed increase in the RZV vaccination rate in the rheumatology clinics at the Orlando VA Healthcare System. This initiative requires continuous awareness from the rheumatology providers and nurses to capture those patients that have not been vaccinated against RZV. Future directions include expanding this initiative to other departments that prescribe immunosuppressive therapy.
To cite this abstract in AMA style:
Camargo Macias K, Shahu Khal R, Schmitz A, McCabe K, Kann T, Mosquera M, Komarla A. Improving Recombinant Zoster Vaccination Rates in Patients Receiving Immunosuppressive Therapy in the Rheumatology Clinic at the Orlando VA Healthcare System – a Quality Improvement Project [abstract]. Arthritis Rheumatol. 2023; 75 (suppl 9). https://acrabstracts.org/abstract/improving-recombinant-zoster-vaccination-rates-in-patients-receiving-immunosuppressive-therapy-in-the-rheumatology-clinic-at-the-orlando-va-healthcare-system-a-quality-improvement-project/. Accessed .« Back to ACR Convergence 2023
ACR Meeting Abstracts - https://acrabstracts.org/abstract/improving-recombinant-zoster-vaccination-rates-in-patients-receiving-immunosuppressive-therapy-in-the-rheumatology-clinic-at-the-orlando-va-healthcare-system-a-quality-improvement-project/