Session Information
Date: Tuesday, November 14, 2023
Title: (1827–1839) Fibromyalgia & Other Clinical Pain Syndromes Poster
Session Type: Poster Session C
Session Time: 9:00AM-11:00AM
Background/Purpose: Fibromyalgia syndrome effects 2-8% of the general population and is characterized by specific tender points. FM annual economic impact $12-$14 billion.Current treatments are only effective in 10% of patients.There is a need to develop alternative treatment methods. Photobiomodulation therapy (PBMT is an electrophysical agent that utilizes non-ionizing forms of light that can be used alone or associated to static magnetic field (PBMT-sMF) to promote analgesia in several health conditions. Evidence of the effects of PBMT alone and PBMT-sMF in FM patients is conflicting.We investigated the efficacy of a new PBMT-sMF device versus placebo on FM.
Methods: A randomized placebo-controlled trial, with blinded patients, therapists, and assessors was performed. 90 female (age= 46 years) patients that met ACR FM diagnostic criteria were randomized into either PBMT-sMF (n=45) or placebo (n=45) groups. Patients from both groups received 9 treatment sessions, 3 times a week, for 3 weeks. PBMT-sMF treatment consisted of 60 J per active tender point during each session, Placebo treatment was 0 J per active tender point. Clinical outcomes were collected at baseline, after the 9th treatment (PostTx) and at 4 weeks follow-up post-treatment. Outcome measures included Tender Point Count (TCP); Impact of FM on their life using Fibromyalgia Impact Questionnaire (FIQ); and Pain Intensity (PI) measured with a 0-100 Visual Analog Scale (VAS).A Fischer’s Exact Test for two independent groups was used to compare proportion of successes between groups and an Unpair T-test was used to analyze pain intensity.Patient satisfaction was measured using a Likert scale.
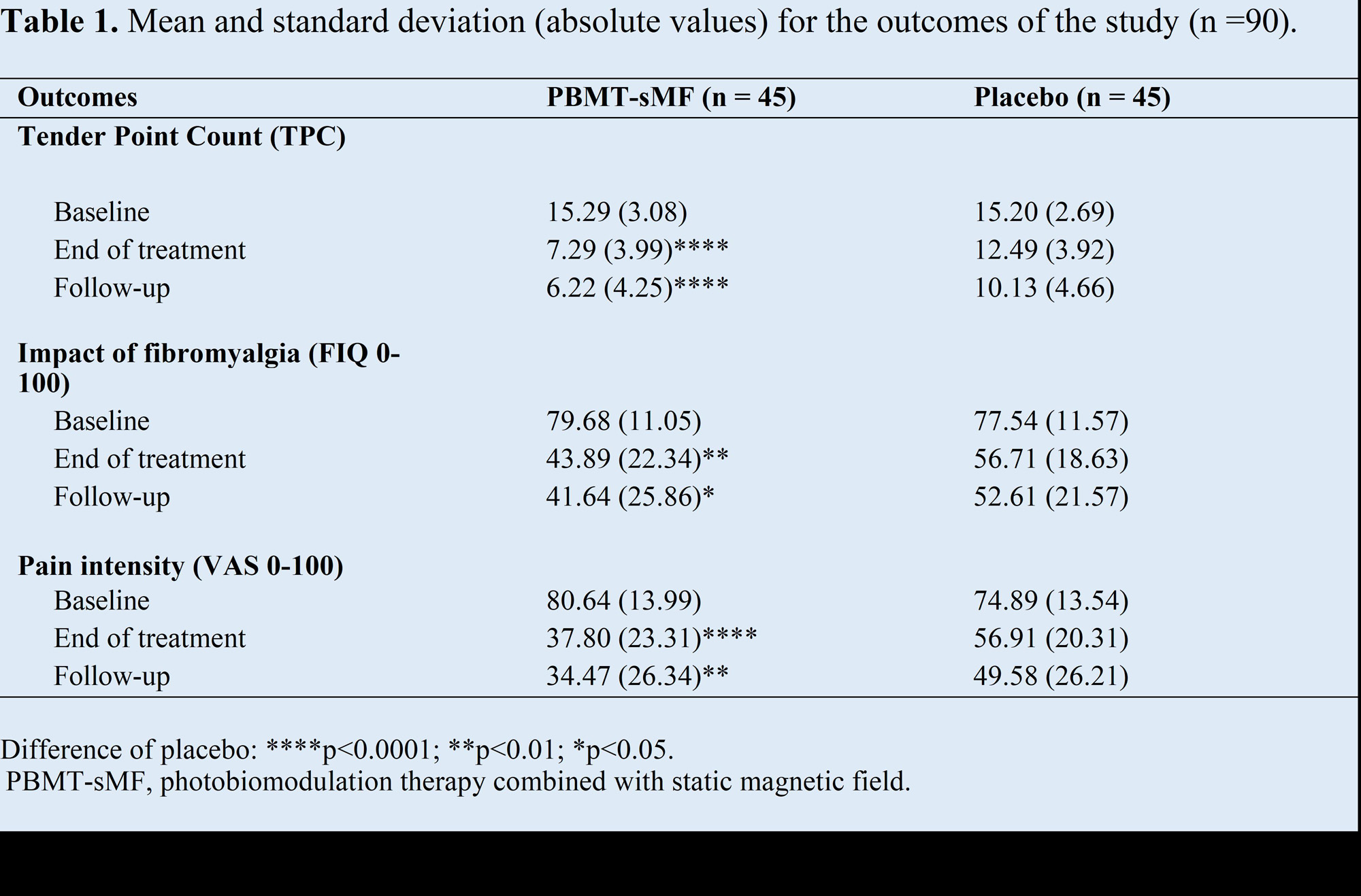
Results: Baseline outcomes measures were equal between groups.PBMT-sMF group reported lower TCP ((p< 0.0001), FIQ score (p< 0.01), and Pain intensity (p< 0.0001) than placebo post treatment.The lower outcomes remained at the 4-week follow-up (TCP p< 0,0001; FIQ p< 0.01; Pain Intensity (p< 0.01)).Table 1 summarizes data and significant differences.TCP decreased 52% Post Tx in PBMT-sMF group versus 18% in Placebo.Additionally, PBMT-sMF group FIQ and Pain intensity decreased 45% and 53% respectively.Placebo FIQ and Pain intensity on decreased 27% and 18% respectively.98% of PBMT-sMF group were somewhat satisfied or satisfied with their outcomes Post-treatment and 91% at the follow-up and the Placebo group were 71% and 73% at same time points.
Conclusion: PBMT-sMF is superior to placebo in decreasing TPC, improving function (decreased FIQ) and overall pain intensity.This study supports using PBMT-sMF to treat patients with fibromyalgia. PBMT-sMF might be considered an important adjuvant to the treatment of patients with fibromyalgia.
To cite this abstract in AMA style:
Demchak T, Leal-Junior E, Ribeiro N, Casalechi H, Johnson D, Tomazoni S. Revolutionizing Fibromyalgia Treatment: Exploring the Efficacy of a New FDA Cleared Photoceutical Device [abstract]. Arthritis Rheumatol. 2023; 75 (suppl 9). https://acrabstracts.org/abstract/revolutionizing-fibromyalgia-treatment-exploring-the-efficacy-of-a-new-fda-cleared-photoceutical-device/. Accessed .« Back to ACR Convergence 2023
ACR Meeting Abstracts - https://acrabstracts.org/abstract/revolutionizing-fibromyalgia-treatment-exploring-the-efficacy-of-a-new-fda-cleared-photoceutical-device/