Session Information
Session Type: Abstract Session
Session Time: 2:00PM-3:30PM
Background/Purpose: Dazodalibep (DAZ) is a non-antibody fusion protein that acts as a CD40L antagonist and blocks costimulatory signals between immune cells, including T cells, B cells, and antigen-presenting cells. Previously, we reported that the primary endpoint, the change from baseline to Day 169 in the EULAR Sjögren’s Syndrome Disease Activity Index (ESSDAI), was achieved in the population of subjects with moderate-to-severe systemic disease activity (NCT04129164).1 Here, we evaluated the efficacy and safety of DAZ therapy in this population through the crossover period of the study.
Methods: We performed a randomized, double-blind, placebo-controlled, crossover study to evaluate DAZ therapy in adult subjects with Sjögren’s having moderate-to-severe systemic disease activity, as defined by an ESSDAI ≥ 5. Eligible subjects were randomized 1:1 to receive intravenous DAZ 1500 mg or PBO Q2W x 3 doses, and then Q4W x 4 additional doses (Stage I). Starting on Day 169, subjects initially randomized to DAZ transitioned to PBO Q4W x 5 doses (DAZ-PBO) and subjects randomized to PBO were switched to DAZ Q4W x 5 doses (PBO-DAZ); all were then followed for 12 weeks (Stage II).
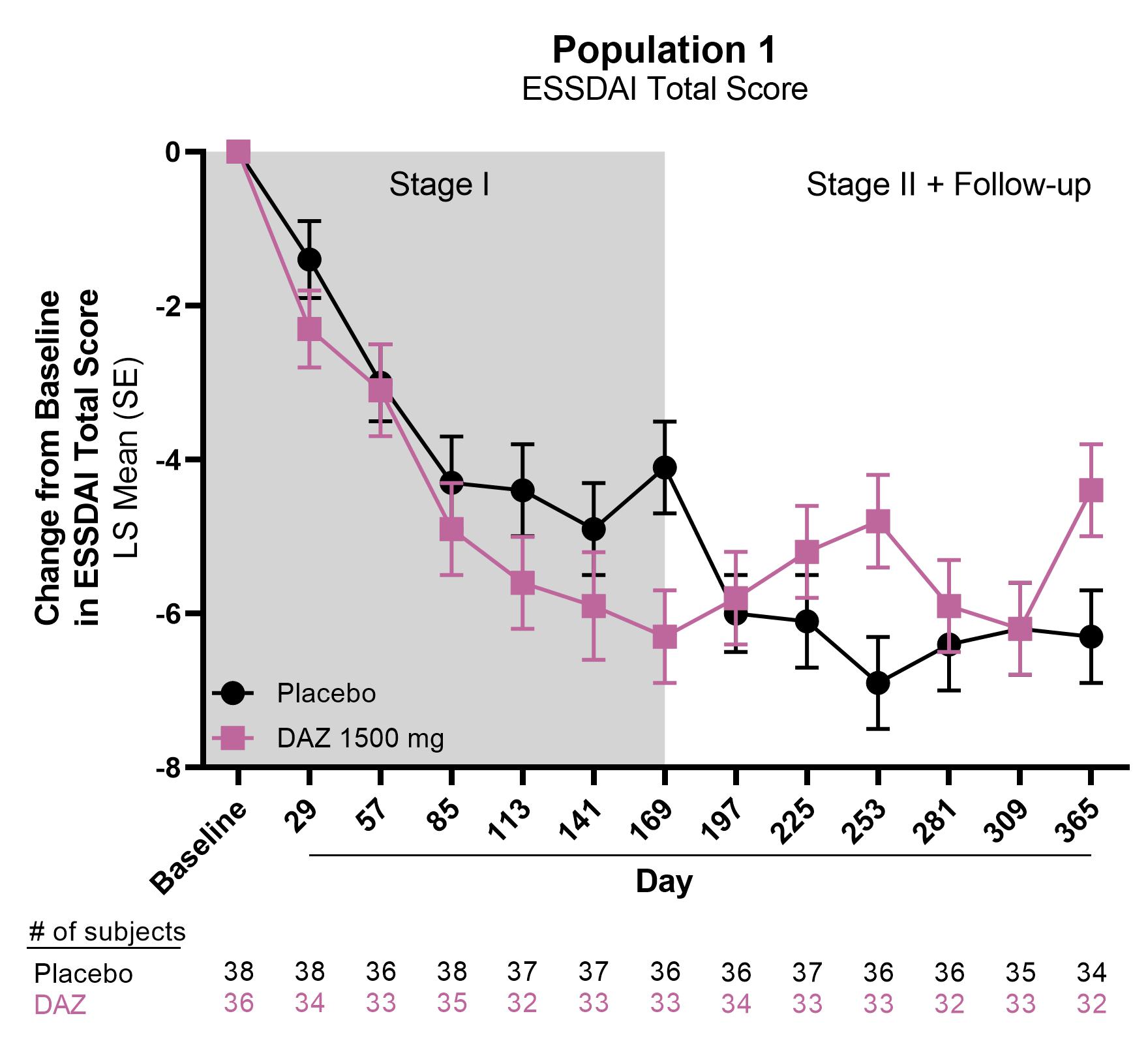
Results: A total of 74 eligible subjects were randomized (DAZ, N=36; PBO, N=38) and received study medication, with 71 (95.9%) completing Stage I and 67 (90.5%) completing Stage II. In the PBO-DAZ group (N=37), the change in ESSDAI score improved from baseline to −4.1 ± 0.6 at Day 169 and to −6.3 ± 0.6 at Day 365 in Stage II. In the DAZ-PBO group (N=34), the change in ESSDAI score was −6.3 ± 0.6 at Day 169 and −4.4 ± 0.6 at Day 365. The proportions of subjects achieving ESSDAI responses (3- or 4-point improvements from baseline) at Day 365 were greater in the PBO-DAZ group compared with the DAZ-PBO group (ESSDAI [3]: 66.7% [24/36] vs 55.9% [19/34]; ESSDAI [4]: 66.7% [24/36] vs 52.9% [18/34]). During Stage II, the PBO-DAZ group showed greater improvement in the EULAR Sjögren’s Syndrome Patient Reported Index, Functional Assessment of Chronic Illness Therapy-Fatigue, and Ocular Surface Disease Index relative to DAZ-PBO group.
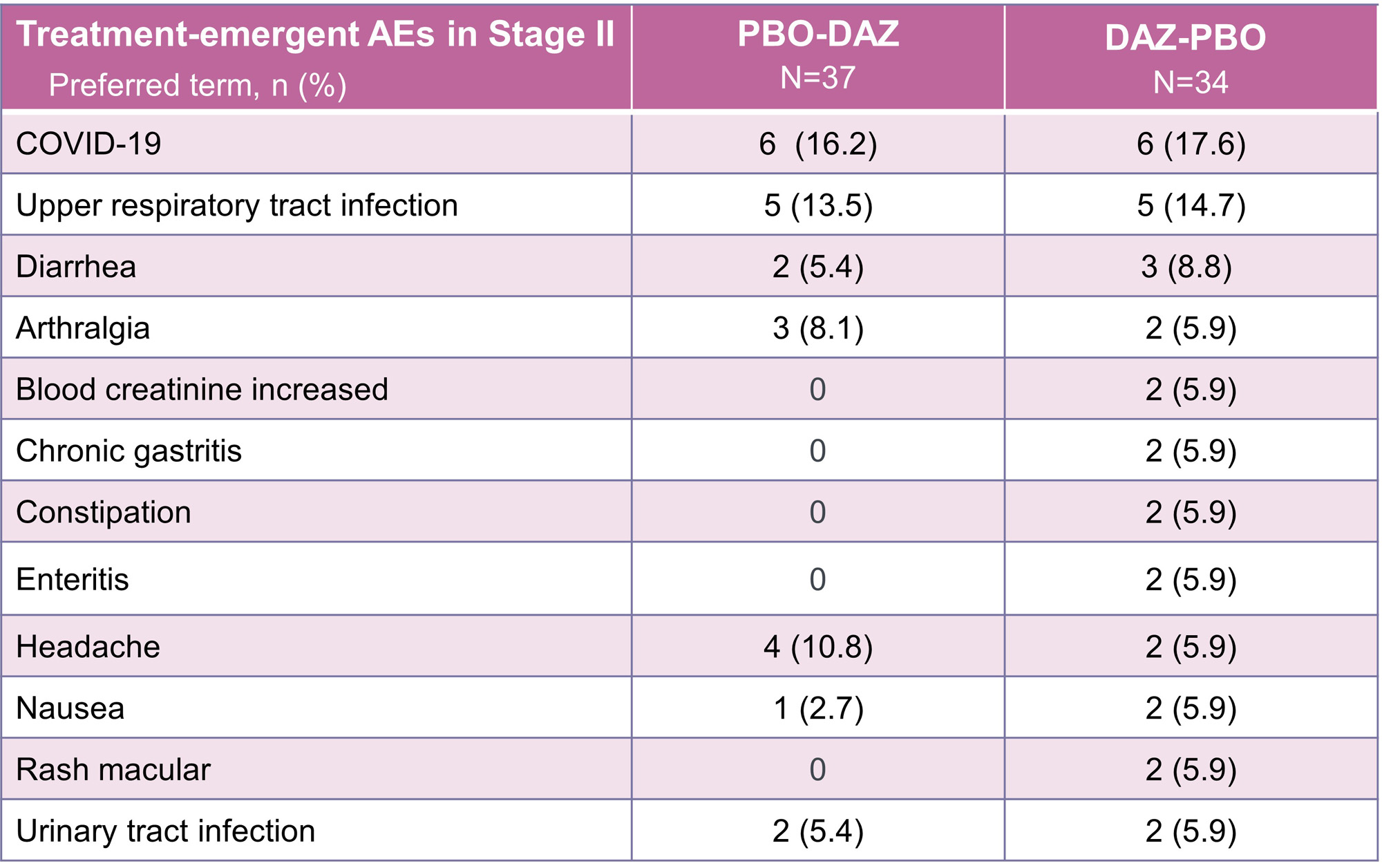
In Stage II, 53 of 71 subjects reported an AE (DAZ-PBO: 25 [73.5%]; PBO-DAZ: 28 [75.7%]) and the majority were mild/moderate in severity. Four SAEs were reported in three subjects in the DAZ-PBO group (drug-induced liver injury and deep vein thrombosis [DVT; also captured as an AESI] in one subject, multiple injuries, and cervical dysplasia). The subject with a lower extremity DVT also had acute liver injury, with onset of SAEs occurring 180 days after the final dose of DAZ. One SAE was reported in the PBO-DAZ group (chronic cholecystitis). One subject in the DAZ-PBO group discontinued the study during Stage II due to an AE (COVID-19) compared to none in the PBO-DAZ group.
Conclusion: The results during Stage II provide further evidence of DAZ clinical efficacy in Sjögren’s disease and support the primary endpoint result. DAZ was generally safe and well tolerated in Stage II, although larger trials of DAZ therapy for this indication are warranted to further explore its safety profile and confirm its clinical efficacy.
References:
1. St. Clair EW et al. Ann Rheum Dis 2023; 82(Suppl 1):95.
To cite this abstract in AMA style:
St. Clair E, Wang L, Alevizos I, Rees W, Baer A, Ng W, Noaiseh G, Baldini C. Dazodalibep, a CD40L Antagonist, in Subjects with Sjögren’s Having Moderate-to-Severe Systemic Disease Activity: Full Crossover Results from a Phase 2, Randomized, Double-Blind, Placebo-Controlled, Proof of Concept Study [abstract]. Arthritis Rheumatol. 2023; 75 (suppl 9). https://acrabstracts.org/abstract/dazodalibep-a-cd40l-antagonist-in-subjects-with-sjogrens-having-moderate-to-severe-systemic-disease-activity-full-crossover-results-from-a-phase-2-randomized-double-blind-placebo-contro/. Accessed .« Back to ACR Convergence 2023
ACR Meeting Abstracts - https://acrabstracts.org/abstract/dazodalibep-a-cd40l-antagonist-in-subjects-with-sjogrens-having-moderate-to-severe-systemic-disease-activity-full-crossover-results-from-a-phase-2-randomized-double-blind-placebo-contro/