Session Information
Session Type: Poster Session B
Session Time: 9:00AM-11:00AM
Background/Purpose: Rapidly progressive diffuse systemic sclerosis (SSc) is a devastating autoimmune disease with high morbidity and mortality. Autologous hematopoietic stem cell transplantation (AHSCT) is recognized as an effective treatment option with randomized controlled trials demonstrating long-term improvement in skin fibrosis, pulmonary function, and overall survival. Although the risk of renal complications following AHSCT for hemato-oncologic conditions has been well established, the risk in SSc remains unclear. In addition to acute kidney injury (AKI), this subset of patients with rapidly progressive, severe SSc is particularly vulnerable to developing scleroderma renal crisis (SRC), a life-threatening complication of SSc characterized by malignant hypertension and AKI.
Methods: We conducted a retrospective review of all patients with SSc treated with AHSCT between 2001 and 2023 at The Ottawa Hospital. All patients satisfied the ACR classification criteria for SSc. Data was sought for baseline patient characteristics and the development of AKI and SRC as defined by the KDIGO 2012 definition or the International Scleroderma Renal Crisis Survey Criteria respectively, during the first 120 days following AHSCT.
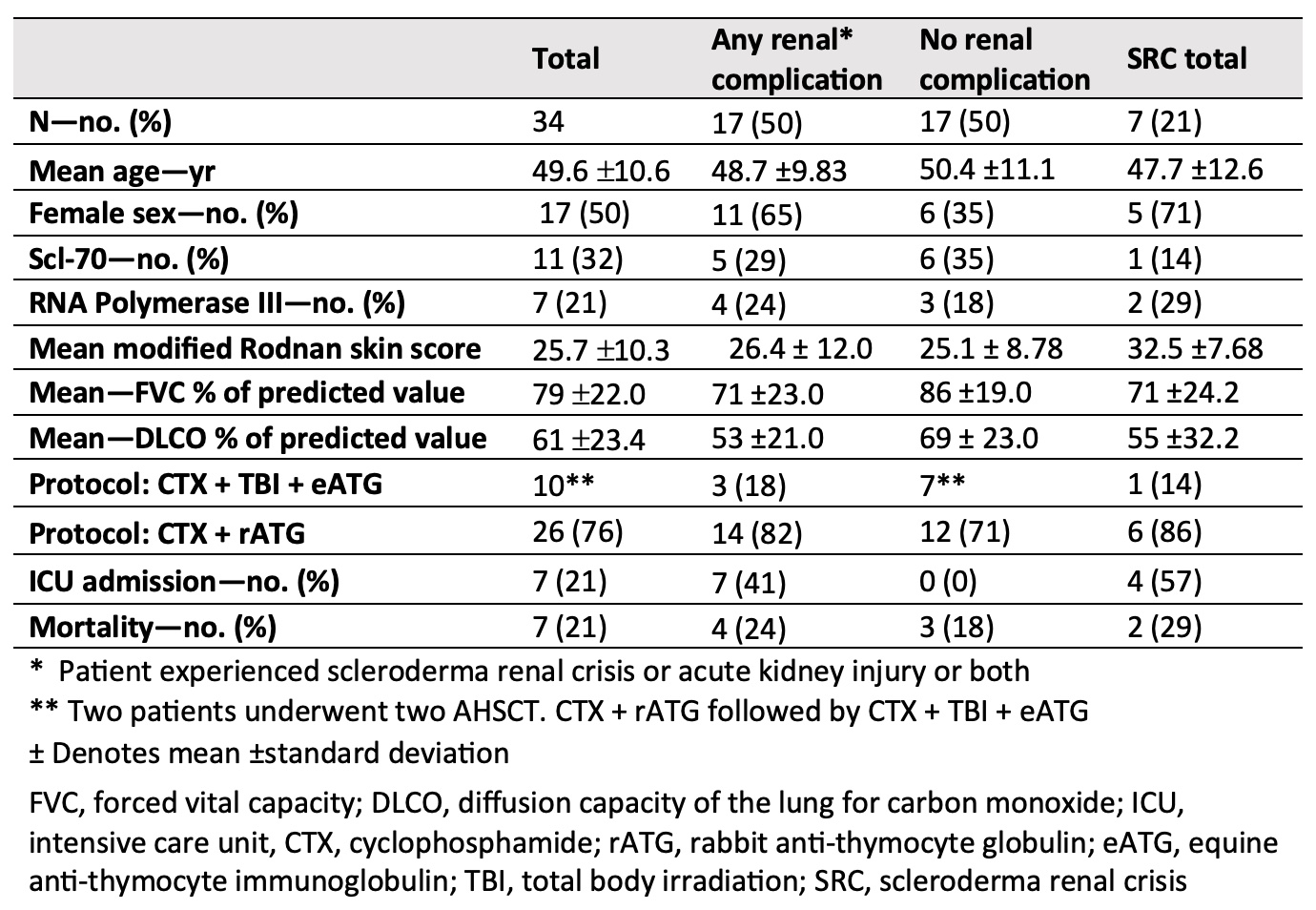
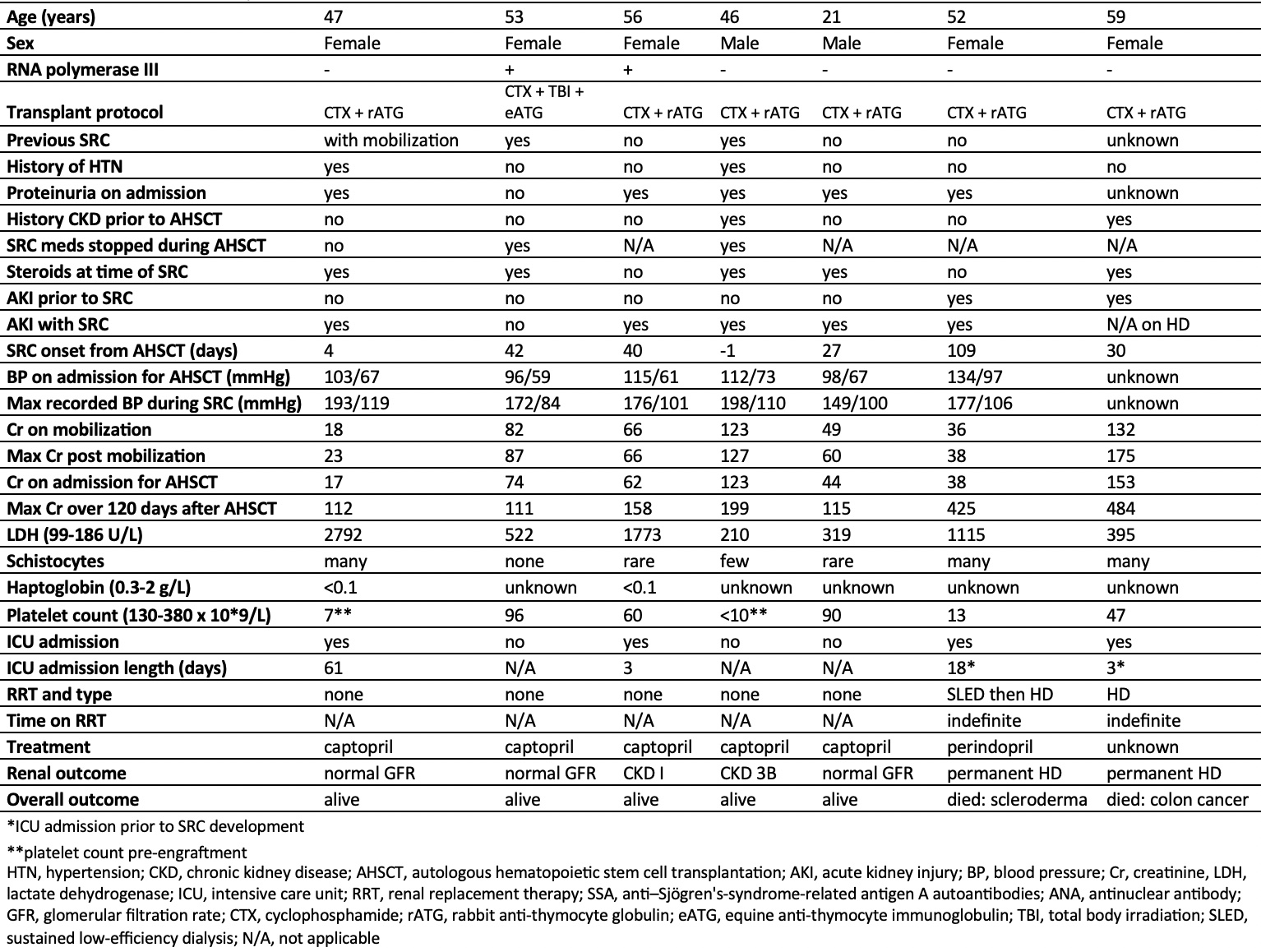
Results: 34 patients underwent AHSCT, the mean age was 49.6 years (21-65), 53% were female, 10 received cyclophosphamide (CTX), equine anti-thymocyte globulin (ATG), total body irradiation (TBI) conditioning and 26 received CTX, rabbit ATG conditioning. The mean modified Rodnan skin score (mRSS) was 25 (2-43), the mean FVC was 79% (37-119) and the mean DLCO was 61% (32-119) before AHSCT. The median follow-up time was 29.9 months (0-72). 50% (n=17) experienced renal complications following AHSCT with 29% (n=10) experiencing AKI alone, 20% (n=7) with SRC alone and 3% (n=1) having SRC without AKI. AKI occurred earlier with a mean of 18 days (-3 to 92) following AHSCT compared to SRC with a mean of 44 days (-1 to 109). 4 of the 7 patients with SRC required admission to the intensive care unit (ICU) and 2 required permanent hemodialysis. Of those with AKI only, 8 out of 10 were diagnosed with pre-renal AKIs and 2 had cardiorenal AKIs. Transient hemodialysis was required in 2 AKI patients. Among those with SRC, 5 patients were receiving steroids at the time of diagnosis. There was no statistically significant difference in overall survival associated with the development of renal complications post-AHSCT.
Conclusion: Although renal complications were common following AHSCT for SSc, affecting half of our cohort, there was no significant impact on survival observed with renal injury in our series. Nonetheless, given the frequency and often the severity of renal morbidity, close monitoring both before and after transplant is warranted as early intervention could be effective. Ideally, with much-needed prospective studies, high-risk patients may be identified prior to AHSCT and provided with pre-emptive supportive interventions to mitigate renal morbidity.
To cite this abstract in AMA style:
MacKenzie M, Atkins H, Maltez N. Renal Complications Following Autologous Stem Cell Transplantation for Systemic Sclerosis [abstract]. Arthritis Rheumatol. 2023; 75 (suppl 9). https://acrabstracts.org/abstract/renal-complications-following-autologous-stem-cell-transplantation-for-systemic-sclerosis/. Accessed .« Back to ACR Convergence 2023
ACR Meeting Abstracts - https://acrabstracts.org/abstract/renal-complications-following-autologous-stem-cell-transplantation-for-systemic-sclerosis/