Session Information
Date: Monday, November 13, 2023
Title: (1412–1441) Spondyloarthritis Including Psoriatic Arthritis – Treatment Poster II: SpA
Session Type: Poster Session B
Session Time: 9:00AM-11:00AM
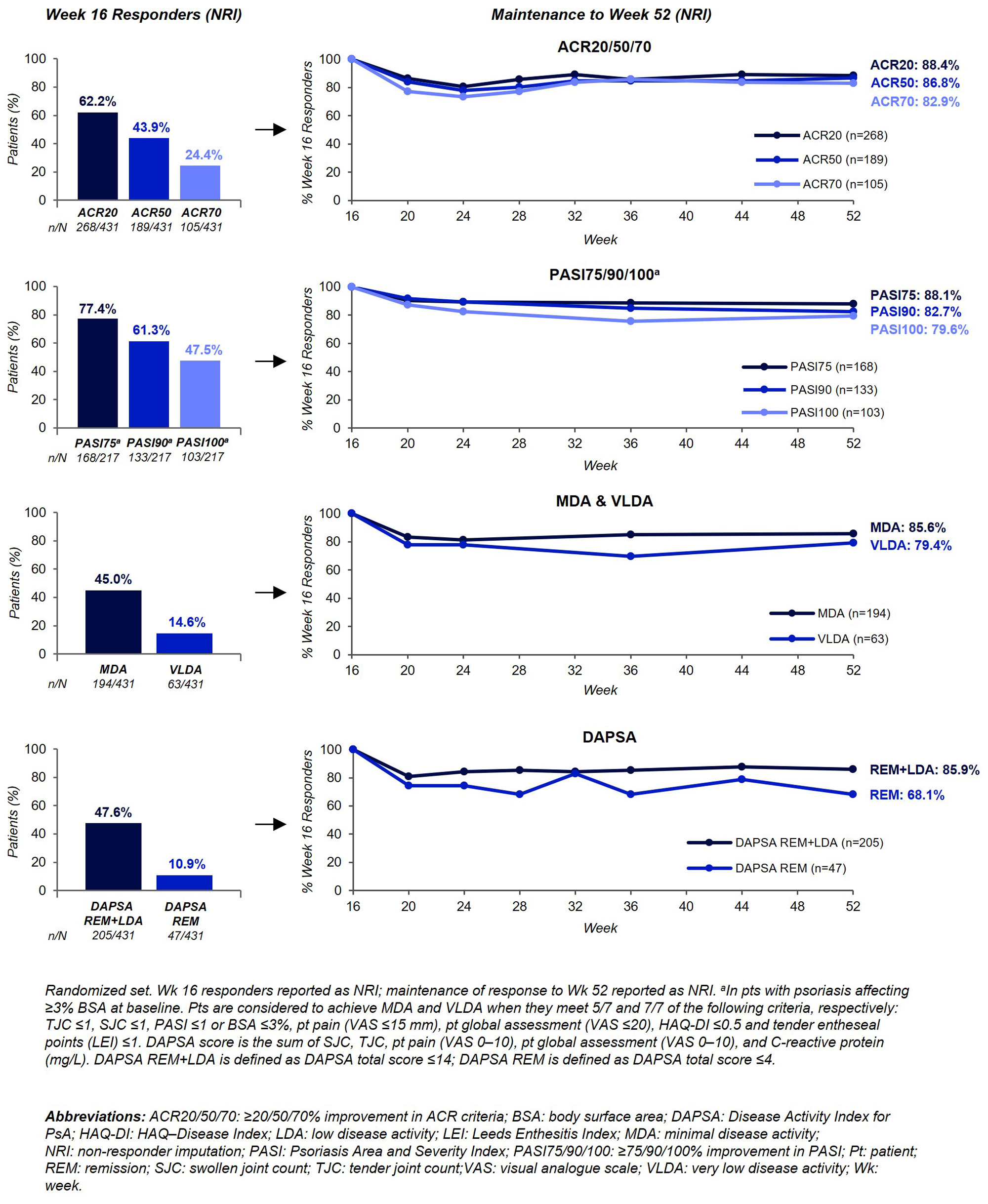
Background/Purpose: Given the chronic nature of PsA, sustaining high levels of disease control with treatment is important. Assessing maintenance of response in patients (pts) that achieve treatment targets is of interest as pts can experience loss of response with long-term therapy.1 Bimekizumab (BKZ), a monoclonal IgG1 antibody that selectively inhibits IL-17F in addition to IL-17A, has demonstrated rapid, clinically meaningful joint and skin efficacy responses at Week (Wk) 16 versus placebo (PBO) in pts with PsA.2,3 Responses were sustained to Wk 52.4 We report maintenance of response in joint and skin efficacy outcomes to Wk 52 in BKZ-treated pts with PsA who responded at Wk 16.
Methods: BE OPTIMAL (NCT03895203), which included a 16-wk double-blind, PBO-controlled period and a 36-wk active treatment-blind period, assessed BKZ in patients with active PsA who were biologic DMARD (bDMARD)-naïve. Pts were randomized 3:2:1 to subcutaneous BKZ 160 mg every 4 wks (Q4W), PBO, or reference (adalimumab 40 mg Q2W). At Wk 16, PBO pts switched to BKZ 160 mg Q4W. Maintenance of response is reported as the percentage of BKZ-treated pts who achieved response at Wk 16 and maintained response at Wk 52. Data are reported for pts randomized to BKZ 160 mg Q4W at baseline (BL). Endpoints include ACR20/50/70, Psoriasis Area and Severity Index (PASI)75/90/100, minimal and very low disease activity (MDA, VLDA), and Disease Activity Index for PsA (DAPSA) remission or low disease activity (REM+LDA; ≤14) and remission (REM; ≤4) responses. Wk 16 responders are reported using non-responder imputation (NRI); maintenance of response to Wk 52 is reported using NRI and observed case (OC). Treatment-emergent adverse events (TEAEs) to Wk 52 are reported for pts who received ≥1 dose of BKZ, including pts randomized to PBO at BL.
Results: At BL, 431 pts were randomized to BKZ 160 mg Q4W; 217/431 (50.3%) had psoriasis affecting ≥3% of body surface area (BSA). 414/431 (96.1%) completed Wk 16; 388 (90.0%) completed Wk 52. Most pts who achieved response at Wk 16 maintained response at Wk 52, across a range of outcomes (Figure). At Wk 16, ACR20/50/70 was achieved by 268 (62.2%)/189 (43.9%)/105 (24.4%) pts; responses were maintained at Wk 52 by 88.4%/86.8%/82.9% (NRI); 92.9%/91.1%/87.9% (OC). Of 217 pts with psoriasis affecting ≥3% of BSA at BL, 133 (61.3%)/103 (47.5%) achieved PASI90/100 at Wk 16. Most pts maintained response at Wk 52: 82.7%, 79.6% (NRI); 94.0%, 89.1% (OC). The same pattern was observed for the composite measures of efficacy. 194 (45.0%) pts achieved MDA at Wk 16; 85.6% (NRI) and 90.7% (OC) maintained response at Wk 52. A high proportion of Wk 16 responders maintained their response at Wk 52 for VLDA, DAPSA REM+LDA, and DAPSA REM (Figure). To Wk 52, 555/702 (79.1%) BKZ-treated pts reported ≥1 TEAE; 46 (6.6%) reported serious TEAEs.
Conclusion: With BKZ treatment, Wk 16 responders maintained robust efficacy responses to Wk 52 across joint, skin, and composite efficacy outcomes. The safety profile of BKZ was consistent with previous reports.2,3
References:1. Boehncke WH. Am J Clin Dermatol 2013;14:377–88; 2. McInnes IB. Lancet 2022; 401(10370):25-37; 3. Merola JF. Lancet 2022; 401(10370):38-48; 4. Ritchlin C. Arthritis Rheumatol 2022;74(suppl 9).
To cite this abstract in AMA style:
Tillett W, Merola J, Tanaka Y, Favalli E, McGonagle D, Walsh J, Thaçi D, Ink B, Bajracharya R, Taieb V, Ritchlin C. Bimekizumab Maintained Efficacy Responses Through 52 Weeks in Biologic Disease-Modifying Antirheumatic Drug-Naïve Patients with Psoriatic Arthritis Who Were Responders at Week 16: Results from a Phase 3, Active-Reference Study [abstract]. Arthritis Rheumatol. 2023; 75 (suppl 9). https://acrabstracts.org/abstract/bimekizumab-maintained-efficacy-responses-through-52-weeks-in-biologic-disease-modifying-antirheumatic-drug-naive-patients-with-psoriatic-arthritis-who-were-responders-at-week-16-results-from-a-phase/. Accessed .« Back to ACR Convergence 2023
ACR Meeting Abstracts - https://acrabstracts.org/abstract/bimekizumab-maintained-efficacy-responses-through-52-weeks-in-biologic-disease-modifying-antirheumatic-drug-naive-patients-with-psoriatic-arthritis-who-were-responders-at-week-16-results-from-a-phase/