Session Information
Session Type: Poster Session B
Session Time: 9:00AM-11:00AM
Background/Purpose: Janus kinase inhibitors (JAKi) have demonstrated substantial efficacy in decreasing symptoms and in reducing progressive joint damage in patients with rheumatoid arthritis (RA). In light of the ORAL Surveillance trial, which was published in January 2021, all JAKi (tofacitinib, baricitinib, upadacitinib) now carry black box warnings concerning risks of serious cardiac events, thromboembolism, cancer, and death associated with these agents. The purpose of this study was to determine whether prescribing practices for Janus kinase inhibitors (JAKi), TNF inhibitors (TNFi), and non-TNFi biologic agents changed after the results of the ORAL Surveillance trial were released in January 2021.
Methods: This is a retrospective study in adult patients with RA receiving advanced therapies within the Veterans Affairs (VA) Health System from January 2012 through September 2022. Eligible patients were required to have at least one diagnosis code for RA and to have received a biologic DMARD or JAKi. Treatment courses were defined from pharmacy dispensing data and the proportion of new courses of each advanced therapy was quantified over time. We assessed changes in the use of each therapy before and after the release of safety data (January 2021).
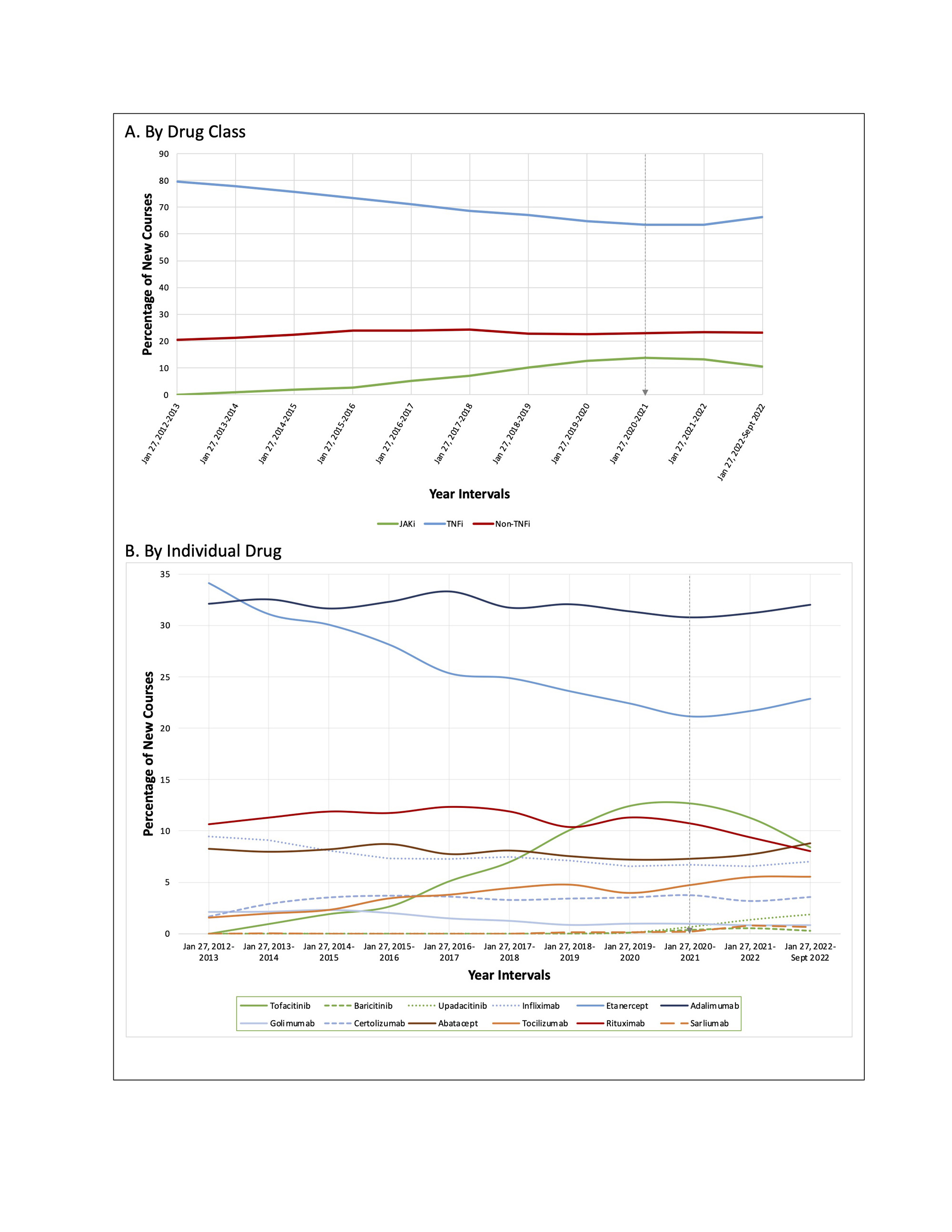
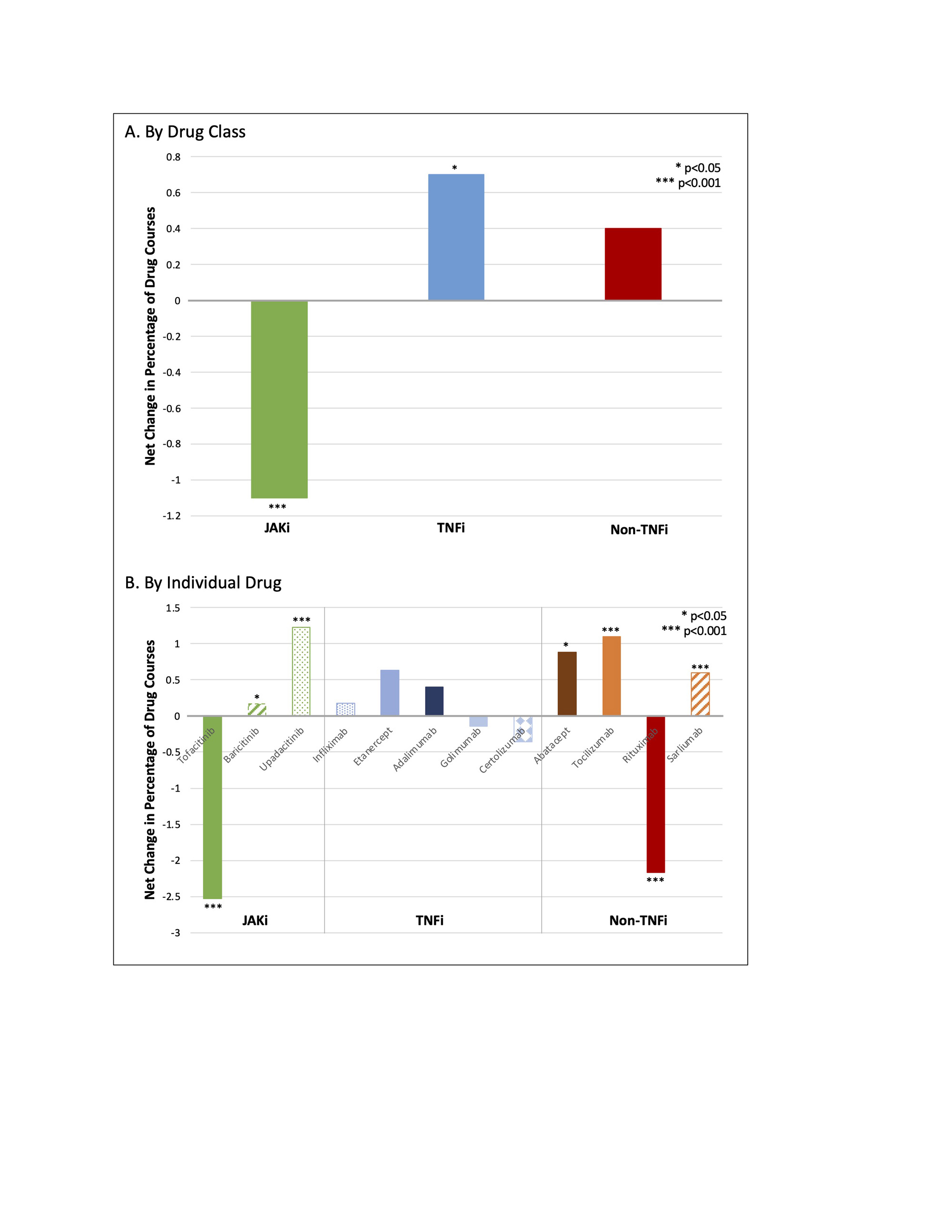
Results: A total of 88,253 individual drug courses (in 34,656 unique patients) were included. The overall number of new drug courses decreased for most of the 11 advanced therapies in 2020, corresponding to the start of the COVID-19 pandemic, before resuming similar levels in 2021. From January 2021 through December 2020, there were consistent increases in the number and proportion of new courses of JAKi, which was followed by a significant net decrease in the proportion of JAKi use through September 2022 (Figure 1A). There were proportionally fewer initiations of tofacitinib after the release of safety data, with a significant difference in the slope of change with time (Figure 1B). In contrast, while use of TNFi declined leading up to 2021, TNFi use significantly increased after January 2021 (Figures 1A and 2A). The number and proportion of prescribed non-TNFi biologics were more variable over these years, with fluctuations in prescribing patterns observed as a class (Figure 2B).
Conclusion: The results of this study suggest that the release of safety data in January 2021 regarding adverse effects of JAKi influenced providers’ prescribing practices for patients with RA. Changes in prescribing in response to new evidence emphasize the impact that safety trials have on prescribing practices. Ongoing study in this area, with attention to specific patient characteristics and risk profiles, will help characterize these changes in practice.
To cite this abstract in AMA style:
Song S, George M, Mikuls T, England B, Sauer B, Cannon G, Baker J. Recent Trends in Treatment Patterns for Rheumatoid Arthritis in Response to Emerging Data [abstract]. Arthritis Rheumatol. 2023; 75 (suppl 9). https://acrabstracts.org/abstract/recent-trends-in-treatment-patterns-for-rheumatoid-arthritis-in-response-to-emerging-data/. Accessed .« Back to ACR Convergence 2023
ACR Meeting Abstracts - https://acrabstracts.org/abstract/recent-trends-in-treatment-patterns-for-rheumatoid-arthritis-in-response-to-emerging-data/