Session Information
Session Type: Poster Session B
Session Time: 9:00AM-11:00AM
Background/Purpose: Due to the improved management strategies and availability of biologic disease-modifying antirheumatic drugs (bDMARDs), ~60% of the rheumatoid arthritis (RA) patients will achieve sustained remission. To ensure optimal rheumatic care, effective use of bDMARDs is required, which includes tapering for RA patients with stable low disease activity. However, previous tapering trials for TNF inhibitors (TNFi) in RA reported that 51-77% of patients experience a disease flare during tapering and stopping. For personalized tapering approach therapeutic drug monitoring (TDM) might be of added value. TDM is based on pharmacokinetics that uses measurements of circulating drug level to adjust the dose individually. Unfortunately, current knowledge on pharmacokinetics of TNFi during tapering is incomplete, e.g. what is the minimal drug level that should be aimed for to prevent disease flares. We aimed to assess the serum level of etanercept (ETN) and adalimumab (ADL) in well-controlled RA patients with and without a disease flare during tapering.
Methods: All RA patients who participated in the TApering strategies in Rheumatoid Arthritis (TARA) trial and followed the tapering protocol and had serum samples from ≥3 visits (n=111) were selected. The TARA trial was a multicenter, single-blinded randomized trial that included established RA patients with a well-controlled disease, defined as a disease activity score (DAS) ≤2.4 and a swollen joint count ≤1. Eligible patients were randomized into gradual tapering csDMARD (mainly methotrexate) followed by the TNFi (ETN or ADL), or vice versa. Tapering of the TNFi was done every three months by doubling the dosing interval, then cutting the dosage into half, and finally stopping. TNFi serum levels were measured at each 3-monthly visit, if serum samples were available, by a drug tolerant enzyme-linked immunosorbent assay.
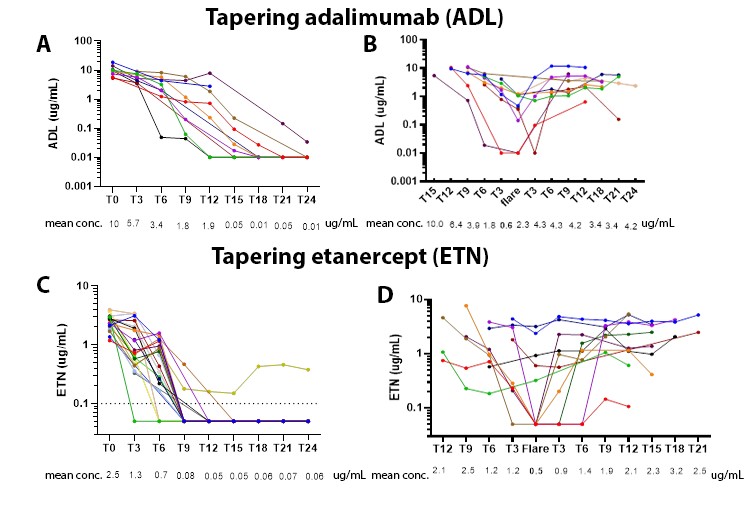
Results: Of the 111 included RA patients 54 tapered their TNFi first, while 57 patients tapered the csDMARD first. The 54 patients who tapered TNFi first had an average symptom duration of 7,2 years and were predominantly female (58%) with an average age of 58 years. At baseline, the DAS (standard deviation) was 1,0 (0,5).Respectively 40% and 60% of the RA patients used ADL and ETN as TNFi. The baseline mean serum concentration for ADL was 7.2 ug/ml (range 0.023-13.4 ug/ml) and 2.7 ug/ml (range 0.5-4.38 ug/ml) for ETN. ADL and ETN serum levels decreased during tapering, but ADL remained longer in the blood circulation after stopping compared to ETN, 6 versus < 3 months respectively (figure 1A and C). Approximately 43% of the patients who tapered the TNFi first flared. At the time of a flare, mean serum level for ADL was 0.6 ug/ml and 0.5 ug/ml for ETN (figure 1B and D).
Conclusion: For both ADL and ETN, the critical serum level below which flares occur seems to be 1 ug/ml. TDM might prevent disease flares during tapering by allowing personalized TNFi dosing targeting levels above this critical threshold. In line with the longer half-live ADL remains longer in the blood circulation after stopping compared to ETN. One should, therefore, be aware that it can take up to 6 months after cessation before the TNFi drops to undetectable levels in blood.
Abbreviations: ADL, adalimumab; conc., concentration; ETN, etanercept
To cite this abstract in AMA style:
Layegh Z, Hooijberg F, loeff F, Dijk l, vaz G, Rispens T, Dolhain r, Wolbink G, de Jong P. Adalimumab and Etanercept Serum Levels in Rheumatoid Arthritis Patients with and Without a Disease Flare During Tapering [abstract]. Arthritis Rheumatol. 2023; 75 (suppl 9). https://acrabstracts.org/abstract/adalimumab-and-etanercept-serum-levels-in-rheumatoid-arthritis-patients-with-and-without-a-disease-flare-during-tapering/. Accessed .« Back to ACR Convergence 2023
ACR Meeting Abstracts - https://acrabstracts.org/abstract/adalimumab-and-etanercept-serum-levels-in-rheumatoid-arthritis-patients-with-and-without-a-disease-flare-during-tapering/