Session Information
Session Type: Poster Session B
Session Time: 9:00AM-11:00AM
Background/Purpose: The efficacy of filgotinib (FIL) for treating RA has been demonstrated in clinical trials. Real-world data are valuable to assess patient-reported outcomes such as pain, fatigue and work productivity, which are negatively impacted by RA.
Methods: FILOSOPHY (NCT04871919) is an ongoing Phase 4, prospective, observational, European study that will enroll ~1500 patients aged ≥18 years with moderate to severe active RA, prescribed FIL for the first time and in accordance with the product label in general practice. At Week 1, 2 and 3 and Month 1, 3, 6, 9 and 12, we assessed the proportion of patients with clinically meaningful change from baseline in pain (reduction of ≥ 10 on a visual analog scale [VAS]) and Functional Assessment of Chronic Illness Therapy (FACIT)-Fatigue score (increase of ≥ 4) in advanced therapy (AT)-naïve and -experienced patients. AT-naïve patients had received no prior biologic (b) or targeted synthetic (ts) disease modifying antirheumatic drugs (DMARDs) for RA; AT-experienced patients had received ≥ 1 prior bDMARD or tsDMARD for RA. Work productivity was also assessed at these timepoints using the Work Productivity and Activity Impairment Questionnaire. DAS28-CRP was assessed at baseline and Month 1, 3, 6 and 12. Adverse events (AEs) were recorded.
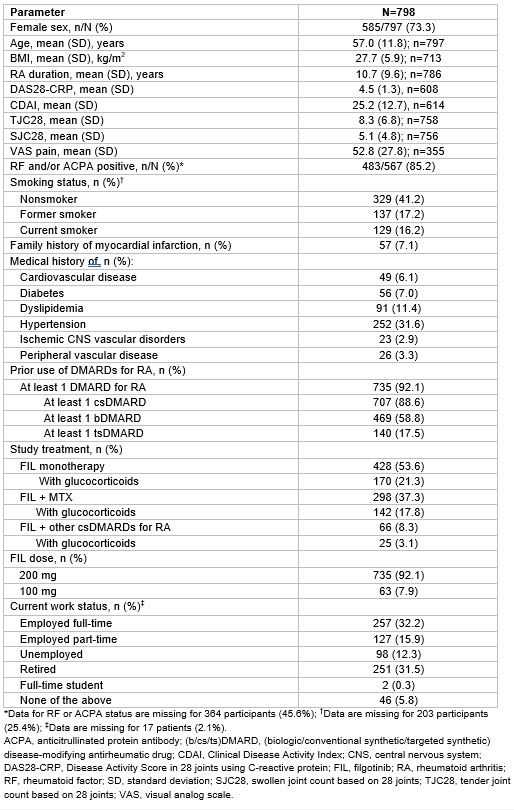
Results: As of Jan 31, 2023, 798 patients had been treated; baseline characteristics are presented in the Table. 53.6% of patients received FIL monotherapy and 37.3% FIL + MTX. 38.2% were AT naïve; 61.8% were AT experienced.
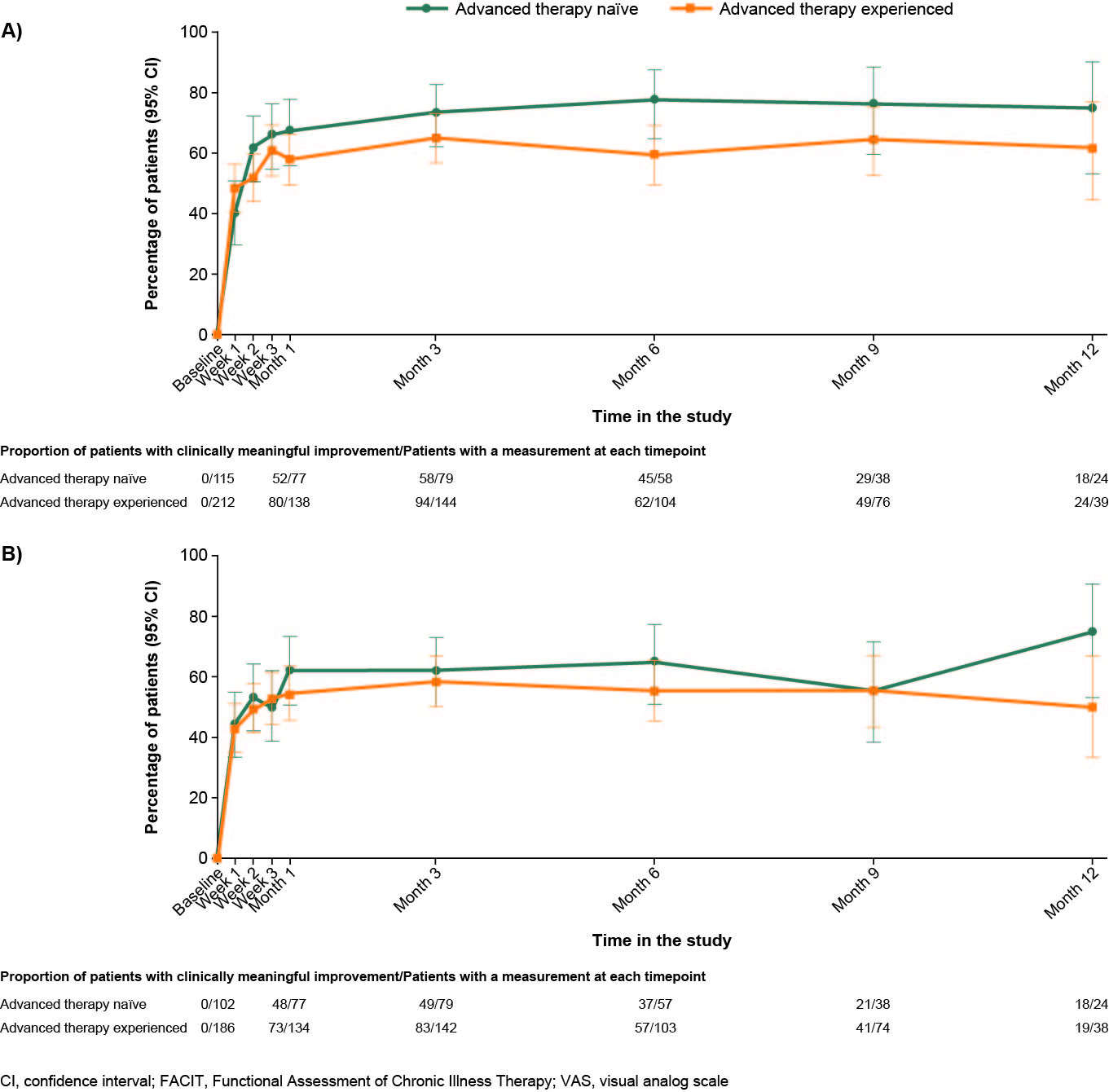
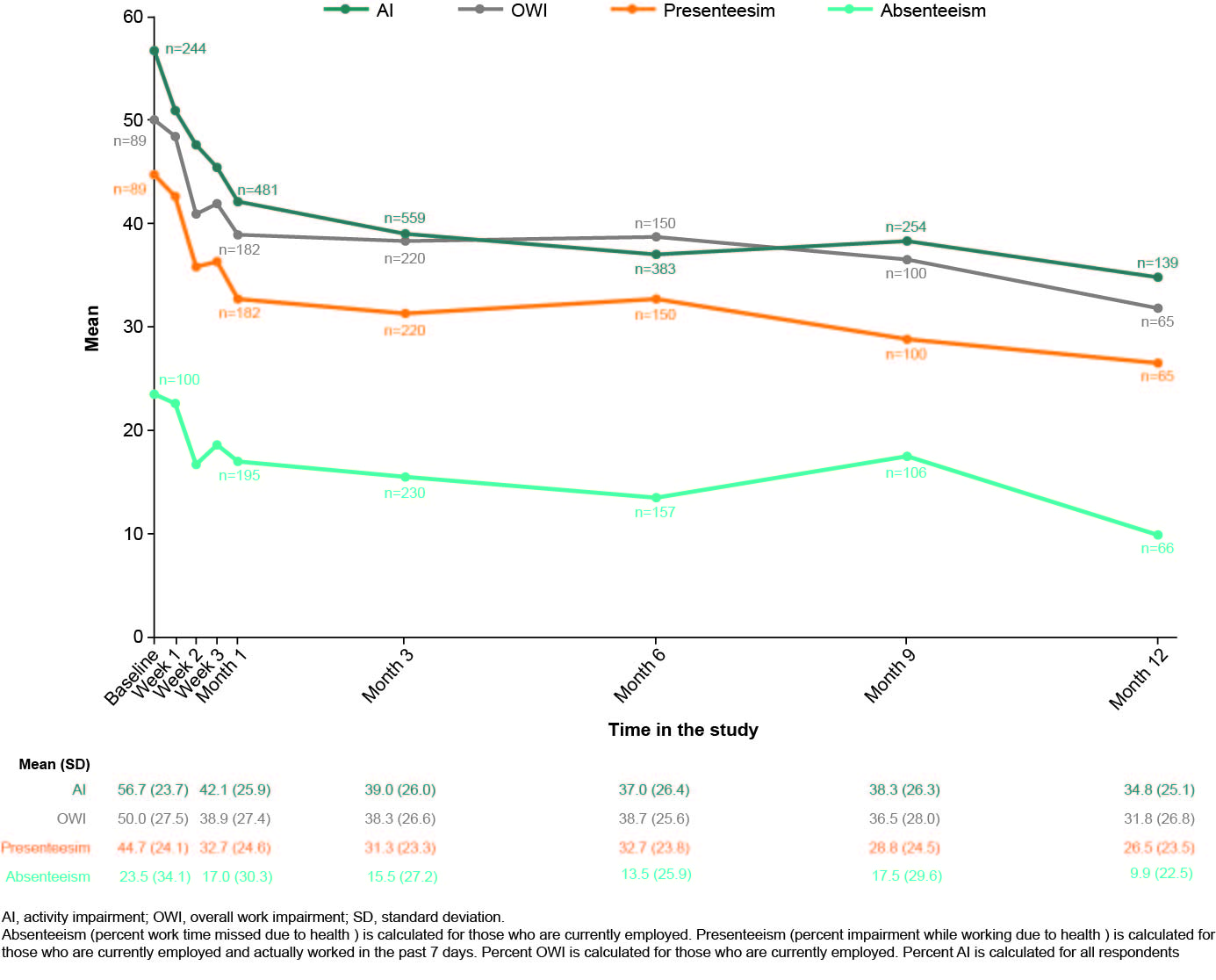
Pain and fatigue improved as early as Week 1. At Week 1, 40.0% of AT-naïve and 48.5% of AT-experienced patients had a clinically meaningful change in VAS pain score (Figure 1A); 44.0% of AT-naïve and 42.9% of AT-experienced patients had a clinically meaningful change from baseline in FACIT-Fatigue (Figure 1B). Clinically meaningful changes in pain and FACIT-Fatigue were maintained until Month 12. Improvements in pain and fatigue were accompanied by changes in work productivity and time spent on daily activities, which increased from as early as Week 1, with further improvements observed until Month 12. Mean (SD) absenteeism decreased between baseline (23.5 [34.1]) and Month 1 (17.0 [30.3]) (Figure 2).
Median DAS28-CRP decreased after 1 month, and decreases were maintained through Month 12. Least squares mean change (SE) from baseline in DAS28-CRP was −1.4 (0.1) to Month 1 and −1.9 (0.1) to Month 12. 43 patients (5.4%) discontinued treatment due to AEs. AEs were mainly infections, including COVID-19 (11.0%), herpes zoster (1.0%), active tuberculosis (0.1%) and opportunistic infections (0.1%). The reported cardiovascular events included stroke (0.5%), transient ischemic attack (0.5%) and unstable angina (0.3%). Neoplasms (excluding NMSC) were reported in 0.8% and fractures in 0.9% of patients. There were 2 deaths.
Conclusion: Interim data from patients treated with FIL show pain, fatigue and work productivity improved as early as Week 1 and DAS28-CRP as early as Month 1, the first timepoint at which DAS28-CRP was assessed; improvements were maintained up to Month 12. No new safety findings were observed up to 12 months. Long-term follow-up is needed to further evaluate effectiveness and safety.
To cite this abstract in AMA style:
Burmester G, Verschueren P, AVOUAC J, Caporali R, Bevers K, Betteridge N, Debray T, De Leonardis F, Romero Yuste S, Zignani M, Galloway J. Patient-Reported Outcomes, Disease Activity and Safety in 798 Patients with RA Treated with Filgotinib: Up to 1-Year Interim Results from a Prospective Observational Study (FILOSOPHY) [abstract]. Arthritis Rheumatol. 2023; 75 (suppl 9). https://acrabstracts.org/abstract/patient-reported-outcomes-disease-activity-and-safety-in-798-patients-with-ra-treated-with-filgotinib-up-to-1-year-interim-results-from-a-prospective-observational-study-filosophy/. Accessed .« Back to ACR Convergence 2023
ACR Meeting Abstracts - https://acrabstracts.org/abstract/patient-reported-outcomes-disease-activity-and-safety-in-798-patients-with-ra-treated-with-filgotinib-up-to-1-year-interim-results-from-a-prospective-observational-study-filosophy/