Session Information
Session Type: Abstract Session
Session Time: 4:00PM-5:30PM
Background/Purpose: Knee osteoarthritis (OA) has unmet need for safe, efficacious symptom and disease-modifying treatments. Lorecivivint (LOR), an intra-articular (IA) CLK/DYRK inhibitor thought to modulate Wnt and inflammatory pathways has previously appeared safe, demonstrated patient-reported outcome (PRO) improvements compared with placebo (PBO), and maintenance of radiographic medial joint space width (JSW). A Phase 3 extension study, OA-07 (NCT04520607), evaluated LOR safety and efficacy with outcomes of medial JSW (mm), Pain Numerical Rating Scale (NRS [0-10]), WOMAC Pain [0-100], and WOMAC Function [0-100].
Methods: Participants had structurally advanced knee OA (medial JSW 1.5-4 mm) and completed the parent Phase 3 trial. A repeat injection according to original randomization (LOR / PBO) was given at the beginning of the single-blind (participants and investigators) extension Year 1. In Year 2 of the extension, all participants received open-label 0.07 mg LOR, while still blind to original treatment assigments. Baseline-adjusted ANCOVA was used to estimate differences between LOR and PBO outcomes using the OA-07 baseline. Treatment effect at Month 36 was estimated using marginal comparison from baseline-adjusted ANCOVA to last PBO observation prior to crossover for LOR only. Concordance between OA-07 baseline-adjusted medial JSW change and pain response was conducted for 36 month completers using logistic regression. Final data are expected Q4 2023.
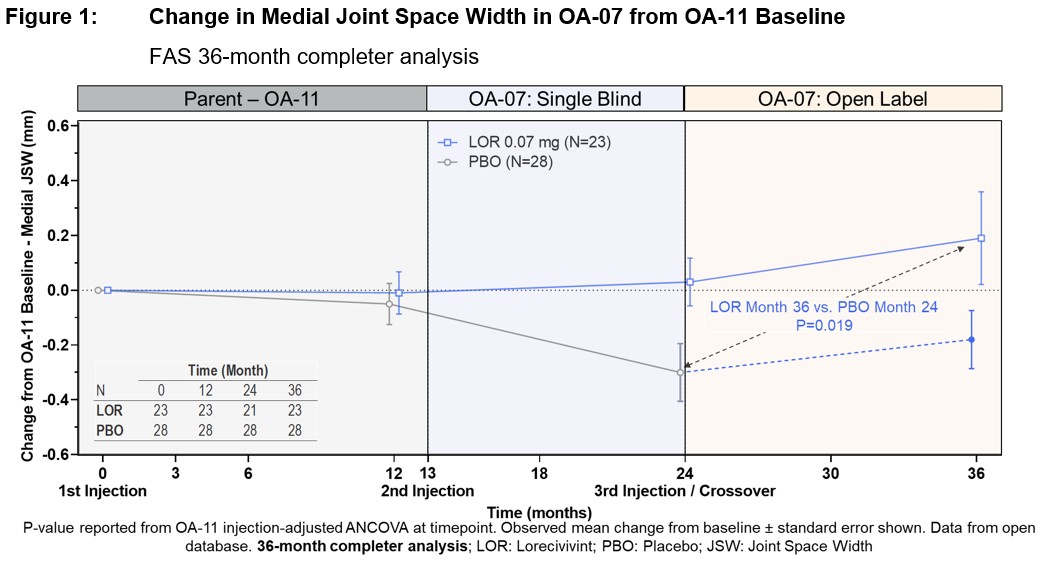
Results: 277 participants (mean age 61.0 ± 8.2 years, BMI 31.8 ± 4.9 kg/m2, female 62.8%, KL3 45.5%, medial JSW 2.63 ± 0.69 mm, 67.1% bilaterally symptomatic, 68.6% medial JSW < 3 mm) were enrolled. LOR appeared safe and well-tolerated. At 24 months, LOR showed numerically reduced medial JSW loss compared to placebo: LOR -0.11 (± 0.05) mm (n=111) vs. PBO -0.20 (± 0.05) mm (n=119) (∆=0.09 mm, 95% CI [-0.06, 0.23], P=0.246). At 36 months, LOR completers (n=23) medial JSW change was +0.19 (± 0.14) mm, difference -0.49 mm (P=0.019) LOR compared to last PBO measure before crossover at 24 months (Figure 1).
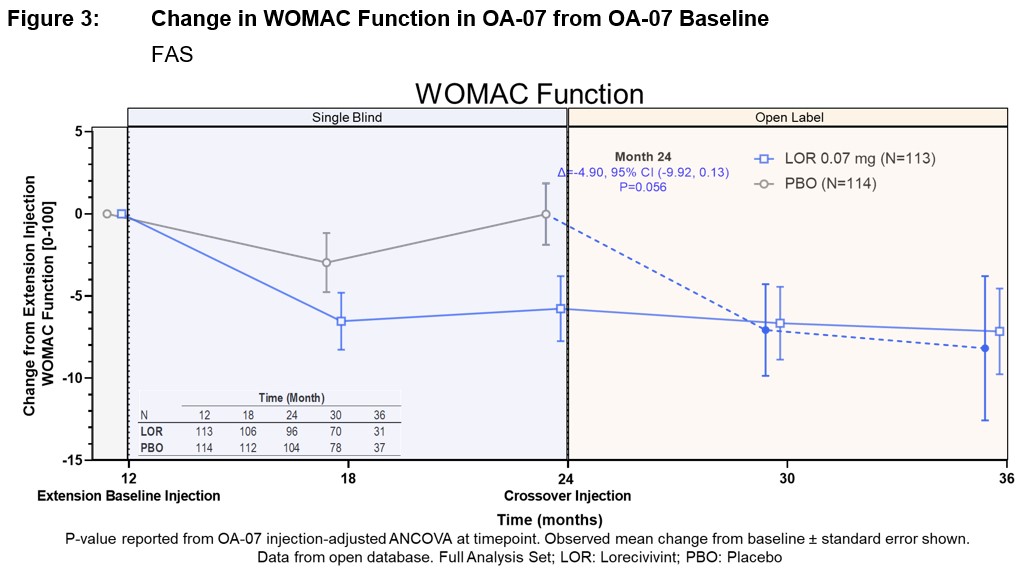
Average change from extension baseline to 24 months in Pain NRS was LOR -0.25 (± 0.19, n=121) compared to PBO 0.09 (± 0.19, n=130) (∆=-0.34, 95% CI [-0.87, 0.19], P=0.207). Similar trends were seen for LOR treatment effect over PBO at 24 months for WOMAC Function ∆=-4.90 (95% CI [-9.92, 0.13], P=0.056) and WOMAC Pain ∆=-5.18 (95% CI [-10.28, -0.08], P=0.047). Additional improvements were seen at 36 months in LOR (n=35) Pain NRS with change from OA-07 baseline of -0.91 (±0.34) and cross-over from PBO to LOR (n=45) improving -0.43 (±0.30). Good concordance was shown between change in medial JSW and at least a 20% improvement in Pain NRS at 36 months (n=20, AUC=0.719).
Conclusion: LOR continued to appear safe and well tolerated. A potential benefit of LOR 0.07 mg compared with PBO in medial JSW was observed 12 months after the extension baseline (second) injection, which persisted in those who completed an additional 12 months (total 36 months, 3 injections) and in PBO participants who crossed to LOR treatment. Potential LOR benefit compared to PBO is also seen across PROs with preliminary good concordance between medial JSW and pain improvement.
To cite this abstract in AMA style:
Yazici Y, Swearingen C, Lopez V, Britt J, Kennedy S, Tambiah J, McAlindon T. Radiographic and Pain Outcomes from a Phase 3 Extension Study Evaluating the Safety and Efficacy of Lorecivivint in Subjects with Severe Osteoarthritis of the Knee (OA-07): 36 Month Single Blind and Placebo Crossover Phase Results [abstract]. Arthritis Rheumatol. 2023; 75 (suppl 9). https://acrabstracts.org/abstract/radiographic-and-pain-outcomes-from-a-phase-3-extension-study-evaluating-the-safety-and-efficacy-of-lorecivivint-in-subjects-with-severe-osteoarthritis-of-the-knee-oa-07-36-month-single-blind-and-p/. Accessed .« Back to ACR Convergence 2023
ACR Meeting Abstracts - https://acrabstracts.org/abstract/radiographic-and-pain-outcomes-from-a-phase-3-extension-study-evaluating-the-safety-and-efficacy-of-lorecivivint-in-subjects-with-severe-osteoarthritis-of-the-knee-oa-07-36-month-single-blind-and-p/