Session Information
Date: Sunday, November 12, 2023
Title: (0673–0690) Vasculitis – ANCA-Associated Poster I: Treatment Outcomes
Session Type: Poster Session A
Session Time: 9:00AM-11:00AM
Background/Purpose: Eosinophilic granulomatosis with polyangiitis (EGPA) is a small-vessel vasculitis characterized by asthma, hyper-eosinophilia, and progressive multiorgan organ involvement. Mepolizumab (MPZ) is a humanized monoclonal anti-interleukin-5 antibody that can reduce peripheral blood eosinophil. Previous studies demonstrated the efficacy and safety of MPZ for induction of remission in patients with relapsing or refractory EGPA, but the efficacy of MPZ during maintenance therapy in patients with EGPA remains unclear. This study is to clarify the efficacy and safety of MPZ during maintenance therapy compared with standard therapy for improving disease activity, preventing relapse, and reducing glucocorticoid (GC) dose.
Methods: This was a retrospective observational study. This study was approved by the Ethics Committees at Toho University School of Medicine (approval number; A22065). Patients, diagnosed with EGPA and treated at Toho University Sakura or Omori Medical Center between January 2016 and June 2022, were included if they met all of the following criteria: prednisolone ≤ 20 mg/day, Birmingham Vasculitis Activity Score (BVAS) < 10 and administration of MPZ or immunosuppressants after 6 months or more from the onset of EGPA. Participants comprised patients with EGPA on MPZ (MPZ group: n = 16) and those on immunosuppressants (standard-of-care [SoC] group: n= 16). Disease activity was evaluated by BVAS as an index of disease activity and a predictor of the prognosis and outcome in patients with EGPA. GC toxicity was evaluated by GC toxicity index (GTI). We investigated BVAS, MPZ retention rate, absolute eosinophil count, cumulative GC dose and GTI after 12 months of MPZ or other immunosuppressant treatment in patients with EGPA.
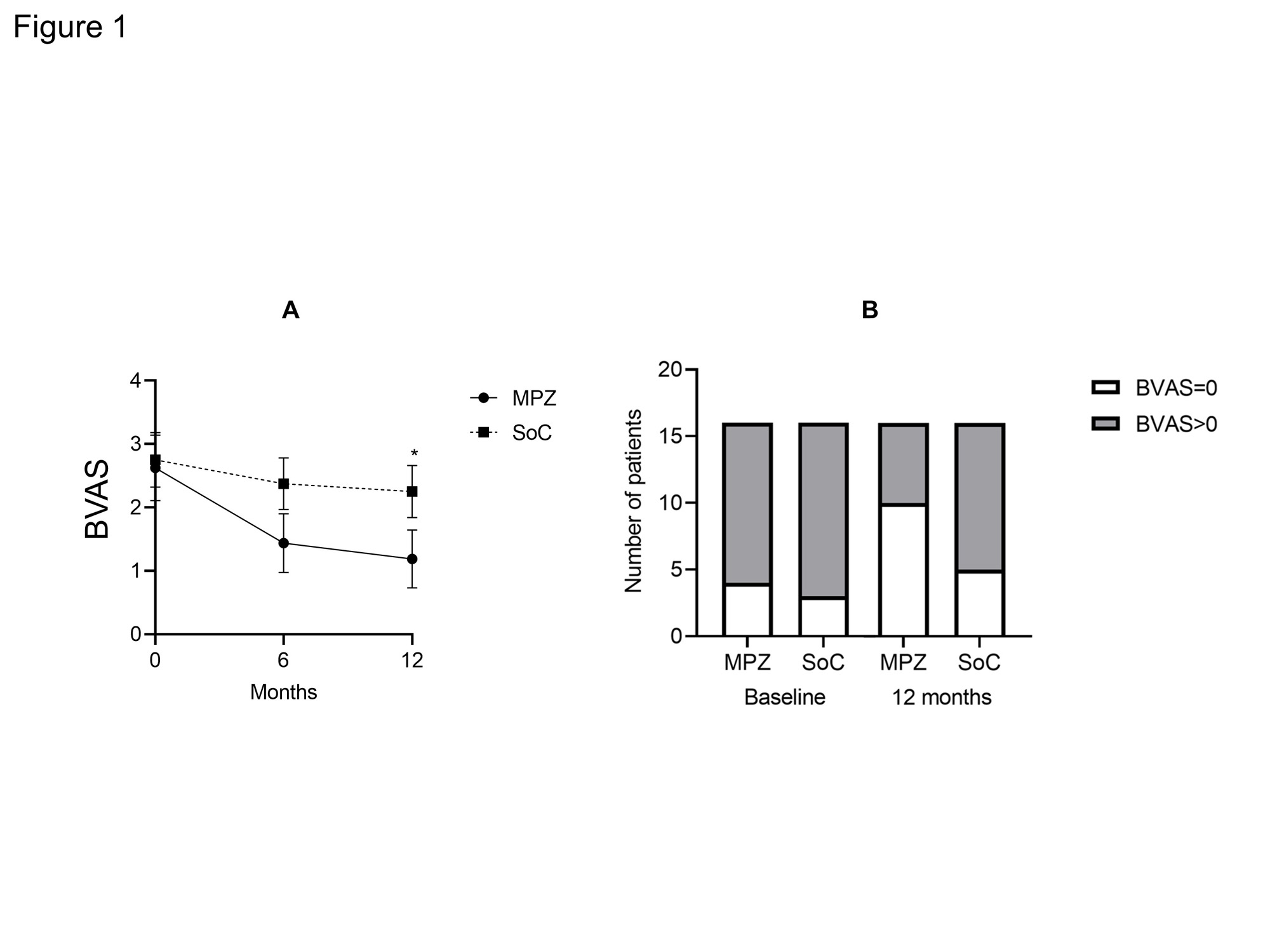
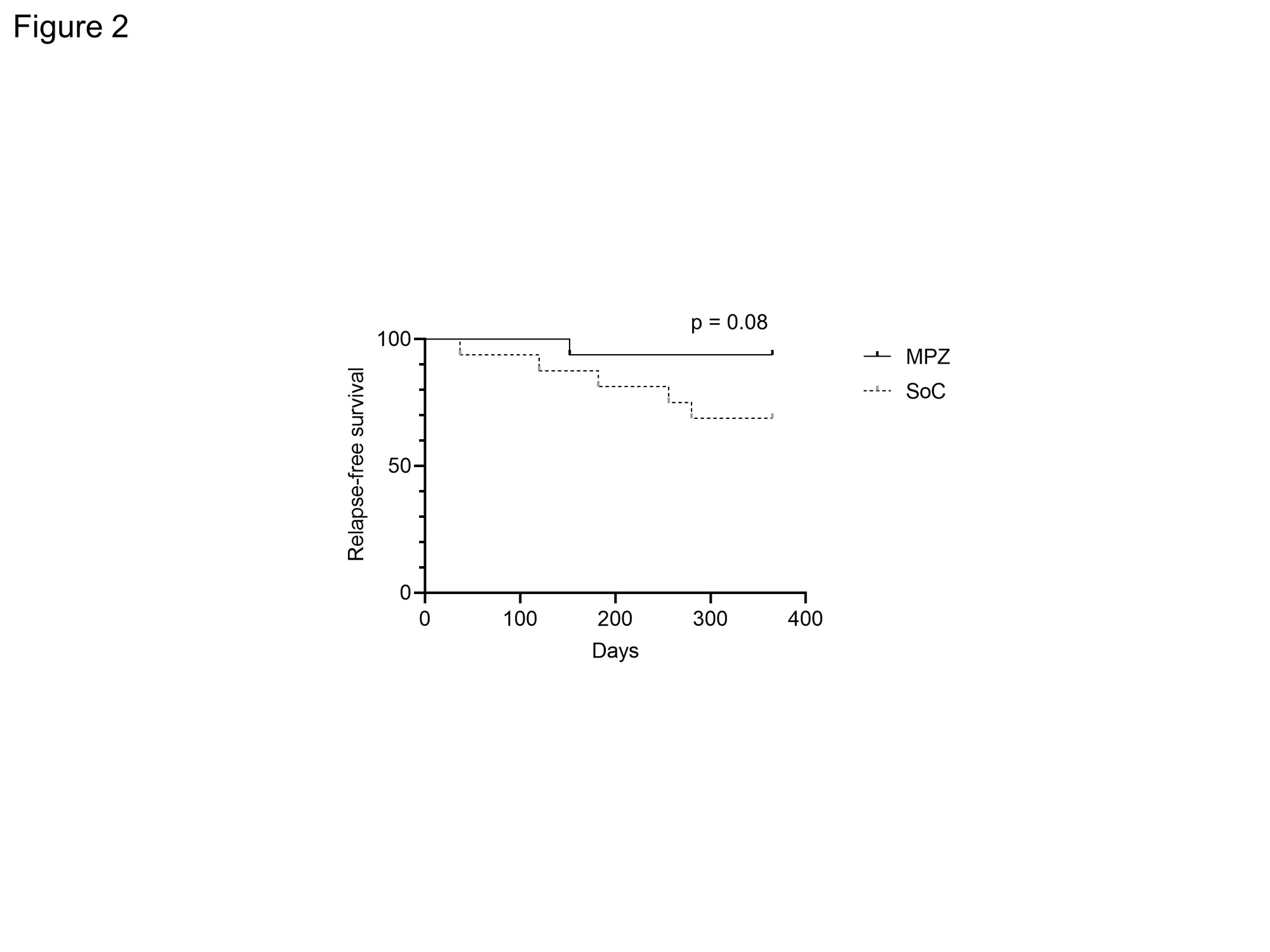
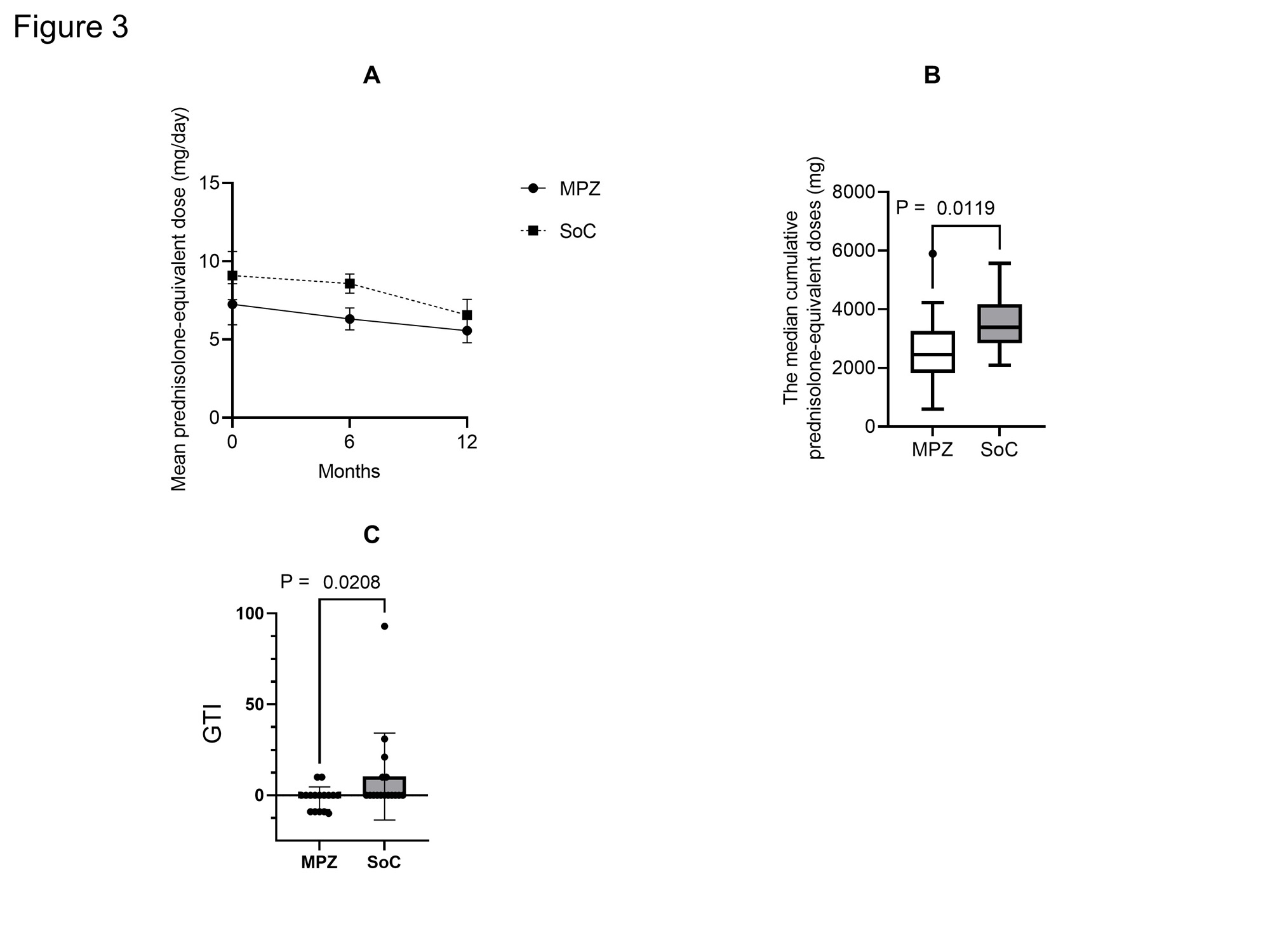
Results: There were no statistically significant differences in age, BMI, CRP, MPO-ANCA, IgE and CRP and BVAS between two groups at baseline. The median disease duration of MPZ group was significantly longer than that of SoC group [46.5 (22.5-122.8) months, 11.0 (3.2-29.0) months, P = 0.001]. The median absolute eosinophil count in MPZ group was significantly higher than that in SoC group [596.0 (114.5-784.2) /μL, 134.6 (2.0-262.1) /μL, P = 0.0034]. No significant differences were observed in the concomitant GC dose or the rate of concomitant immunosuppressant use between the groups. The BVAS at 12 months after treatment was significantly lower in MPZ group than in SoC group (P = 0.0367) (Figure 1). Percentage of patients achieving BVAS = 0 at 12 months tended to be higher in MPZ group than in SoC group. Relapse rates tended to be lower in the MPZ group (1 patient) than in the SoC group (6 patients) (P = 0.08) (Figure 2). Cumulative GC dose at 12 months were significantly decreased in the MPZ group than in the SoC group [2452 (1825-3256) mg, 3384 (2840-4179) mg, P = 0.0356] (Figure 3). The GTI at 52 weeks was significantly lower in the MPZ group than in the SoC group [0.0 (-9.0-0.0), 0.0 (0.0-10.0), P = 0.0208].
Conclusion: Our study demonstrated that administration of MPZ to EGPA during maintenance therapy reduced disease activity and relapse rates. MPZ in EGPA maintenance therapy may also have the potential to reduce cumulative GC dosage and GC toxicity.
To cite this abstract in AMA style:
Sakai D, Kaneko K, Furukawa K, Kawazoe M, Matsuzawa Y, Nanki T. Efficacy and Safety of Mepolizumab During Maintenance Therapy in Patients with Eosinophilic Granulomatosis with Polyangiitis [abstract]. Arthritis Rheumatol. 2023; 75 (suppl 9). https://acrabstracts.org/abstract/efficacy-and-safety-of-mepolizumab-during-maintenance-therapy-in-patients-with-eosinophilic-granulomatosis-with-polyangiitis/. Accessed .« Back to ACR Convergence 2023
ACR Meeting Abstracts - https://acrabstracts.org/abstract/efficacy-and-safety-of-mepolizumab-during-maintenance-therapy-in-patients-with-eosinophilic-granulomatosis-with-polyangiitis/