Session Information
Session Type: Poster Session A
Session Time: 9:00AM-11:00AM
Background/Purpose: SLE is a chronic disease that progressively reduces patients’ health-related quality of life.1 In a post hoc analysis of the TULIP trials, patients with SLE reported improvements in patient-reported outcome measures over 52 weeks.2 Here, we evaluated the long-term impact of anifrolumab treatment on the Short Form 36 Health Survey version 2 (SF-36v2) in patients receiving standard therapy from the placebo-controlled, phase 3 long-term extension (LTE) trial.3
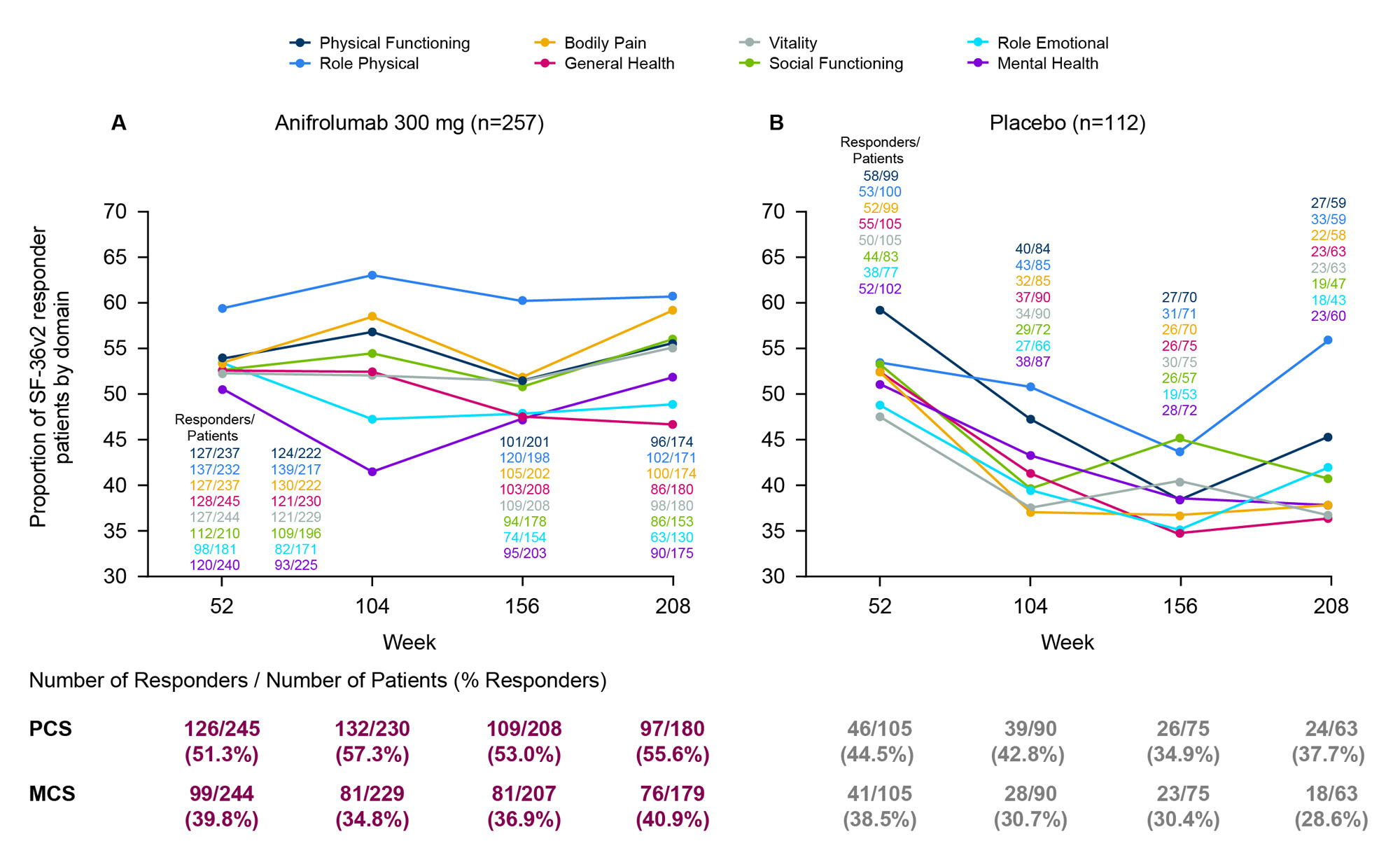
Methods: Adult patients with moderate to severe SLE (1997 ACR criteria) who completed a 52-week TULIP-1 or TULIP-2 trial could enroll in the 3-year LTE study (NCT02794285). Patients included in this analysis were randomized to intravenous anifrolumab 300 mg or placebo every 4 weeks in TULIP-1/-2 and continued in the same treatment group in the LTE, in addition to standard therapy. Proportions of patients classified as responders (defined by positive changes in responses from baseline greater than or equal to the minimum clinically important differences [MCIDs]) in Physical and Mental Component Scores (PCS/MCS) and SF-36v2 individual domains (acute recall) were analyzed for each treatment group using a Cochran–Mantel–Haenszel approach from Weeks 52 to 208. MCIDs were defined as changes from baseline ≥2.5 for PCS and MCS scores and ≥5 for all SF-36v2 domains.2Patients with baseline PCS ≤73.01 and MCS ≤67.15 scores and those with each baseline SF-36v2 domain score ≤95 were included in each respective analysis. Domain scores were non-normalized and scaled from worst to best (0–100).
Results: The proportion of PCS responders treated with anifrolumab (n=257; Figure A) increased over time from 51% at Week 52 to 56% at Week 208, whereas the proportion of responders decreased in the placebo group (n=112; Figure B) from 45% to 38%. The proportion of MCS responders was sustained for patients on anifrolumab with a 1% increase over 3 years, but decreased by 10% for patients on placebo. Proportions of SF-36v2 responders treated with anifrolumab were generally maintained throughout the 3-year LTE period, with 47%–61% of patients reporting improved domain scores at Week 208. In all SF-36v2 domains except for General Health and Role Emotional, similar or higher proportions of anifrolumab-treated patients were responders at Week 208 compared with Week 52. In contrast, the proportions of responders receiving placebo decreased over time in all domains except Role Physical, ranging from 36%–56% at Week 208.
Conclusion: Patients with SLE treated with anifrolumab had sustained improvements in PCS, MCS, and most SF-36v2 individual domains over the 3-year LTE period. This was not observed in patients on standard therapy alone, despite the higher dropout rates and survivor bias observed in the placebo group.3 These results support the long-term benefit of anifrolumab on self-reported, clinically important, health status outcomes across multiple domains.
References:
1. Kaul A, et al. Nat Rev Dis Primers.2016;2:16039.
2. Strand V, et al. Lancet Rheumatol. 2022;4:e198–e207.
3. Kalunian KC, et al. Arthritis Rheumatol. 2023;75:253–65.
CMH, Cochran–Mantel–Haenszel; GC, glucocorticoids; IFN, interferon; LTE, long-term extension; MCS, Mental Component Score; PCS, Physical Component Score; SF_36v2, Short Form 36 Health Survey version 2; TULIP, Treatment of Uncontrolled Lupus via the Interferon Pathway.
Percentages of responders were calculated using a stratified CMH approach (stratification factors: SLEDAI_2K score at screening [<10 points vs
≥10 points], Week 0 GC dose [<10 mg/day vs ≥10 mg/day] and type I IFN gene signature test result at screening [high vs low]).
Responders were defined as positive changes in responses from baseline greater than or equal to the minimum clinically important differences.
To cite this abstract in AMA style:
Strand V, Kalunian K, Desta B, Seo C, Abreu G, Tummala R, Lindholm C, Al-Mossawi H. Evaluation of Anifrolumab Treatment Responses by the Short Form 36 Health Survey Version 2 in SLE: A Post Hoc Analysis of the Placebo-Controlled Phase 3 Long-Term Extension Trial [abstract]. Arthritis Rheumatol. 2023; 75 (suppl 9). https://acrabstracts.org/abstract/evaluation-of-anifrolumab-treatment-responses-by-the-short-form-36-health-survey-version-2-in-sle-a-post-hoc-analysis-of-the-placebo-controlled-phase-3-long-term-extension-trial/. Accessed .« Back to ACR Convergence 2023
ACR Meeting Abstracts - https://acrabstracts.org/abstract/evaluation-of-anifrolumab-treatment-responses-by-the-short-form-36-health-survey-version-2-in-sle-a-post-hoc-analysis-of-the-placebo-controlled-phase-3-long-term-extension-trial/