Session Information
Date: Sunday, November 12, 2023
Title: (0380–0422) RA – Diagnosis, Manifestations, and Outcomes Poster I
Session Type: Poster Session A
Session Time: 9:00AM-11:00AM
Background/Purpose: Although the use of patient-reported outcome (PRO) data has grown more common in the care of people with rheumatoid arthritis (RA), the utility of PRO data captured between visits in relation to traditional, physician-derived measures has not been adequately evaluated. We compared changes over time in PRO data captured on a smartphone app against a physician-derived disease activity measure, the Clinical Disease Activity Index (CDAI).
Methods: RA patients in moderate or high disease activity (CDAI >10) and prescribed either upadacitinib (UPA) or adalimumab (ADA) were enrolled in the study from March 2021 to October 2022 during in-office visits at 28 community rheumatology clinics in the US. Patients were prompted to download the ArthritisPower registry smartphone app to complete PROs at home over a 12-week period. Following a mandatory run-in period to verify willingness to provide PRO data, subsequent at-home data collection included disease activity measures as well as symptom measures from the Patient-Reported Outcomes Measurement Information System (PROMIS). Patients returned for a follow-up clinical visit at approximately 3-4 months (follow-up visits permitted at 2-6 months). Rheumatology clinics provided baseline clinical data including CDAI, years since RA diagnosis, prior medications, and follow-up CDAI. A subgroup analysis was restricted to those who stayed on medication with minimal interruptions. Linear regression models adjusted for baseline CDAI and baseline PRO scores were used to test the correlation between follow-up CDAI and follow-up PRO scores.
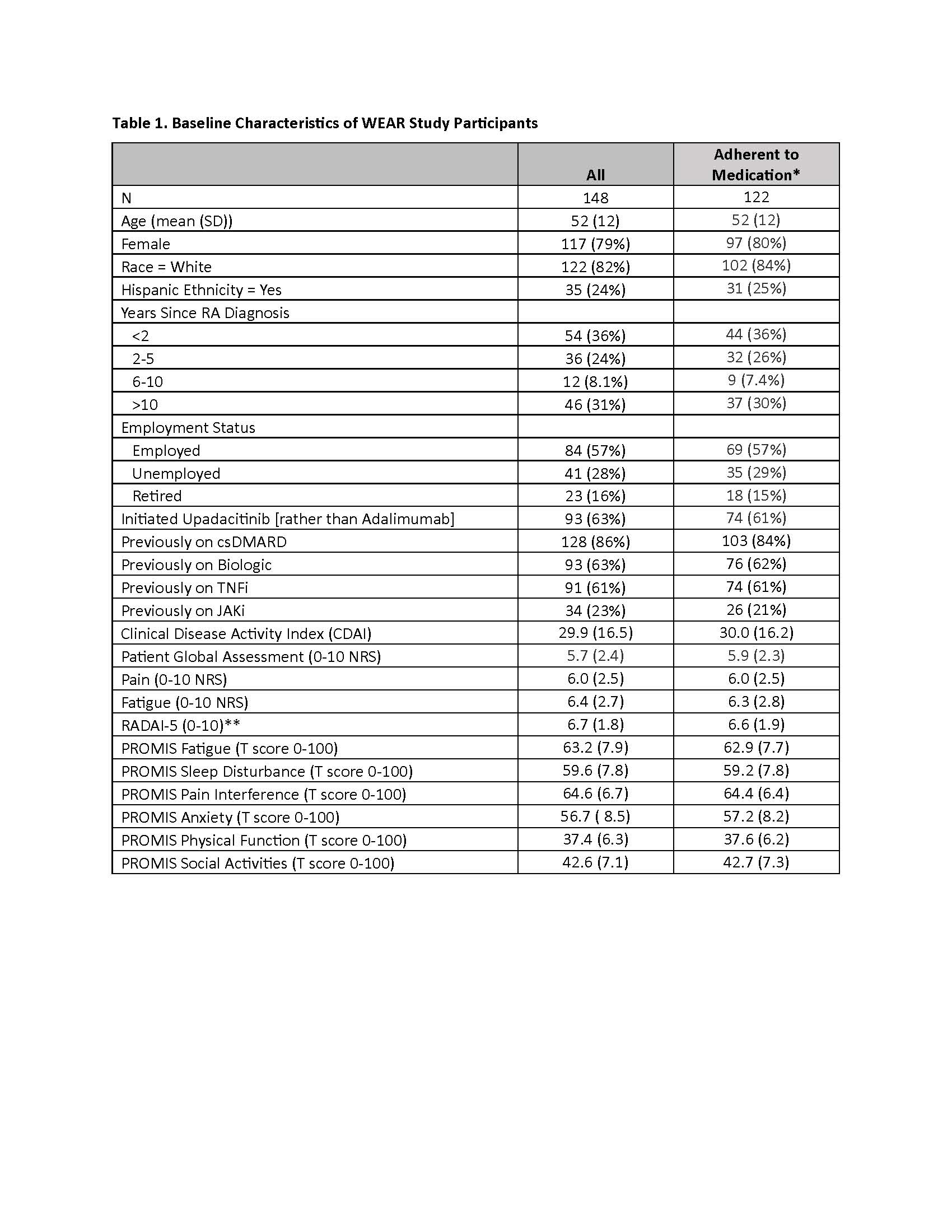
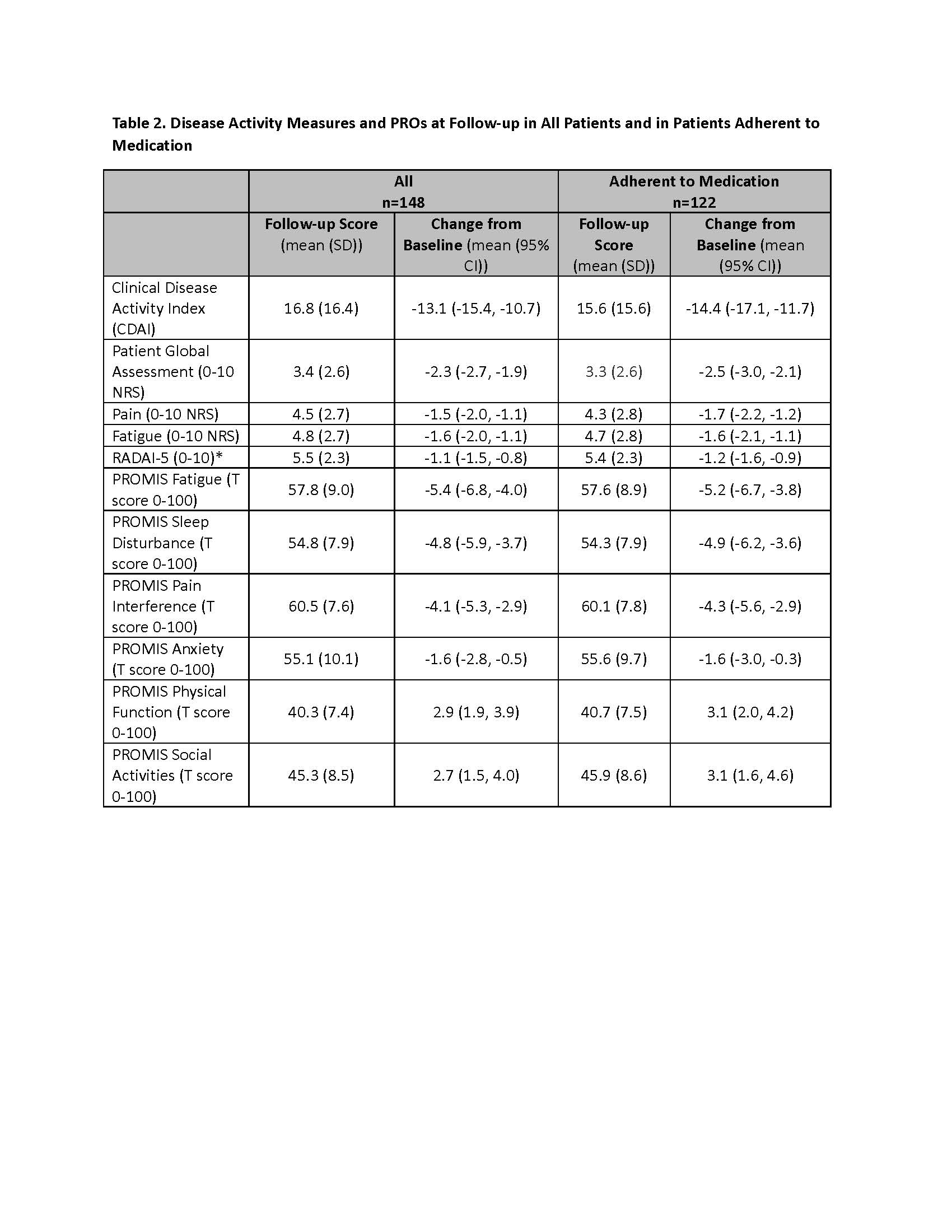
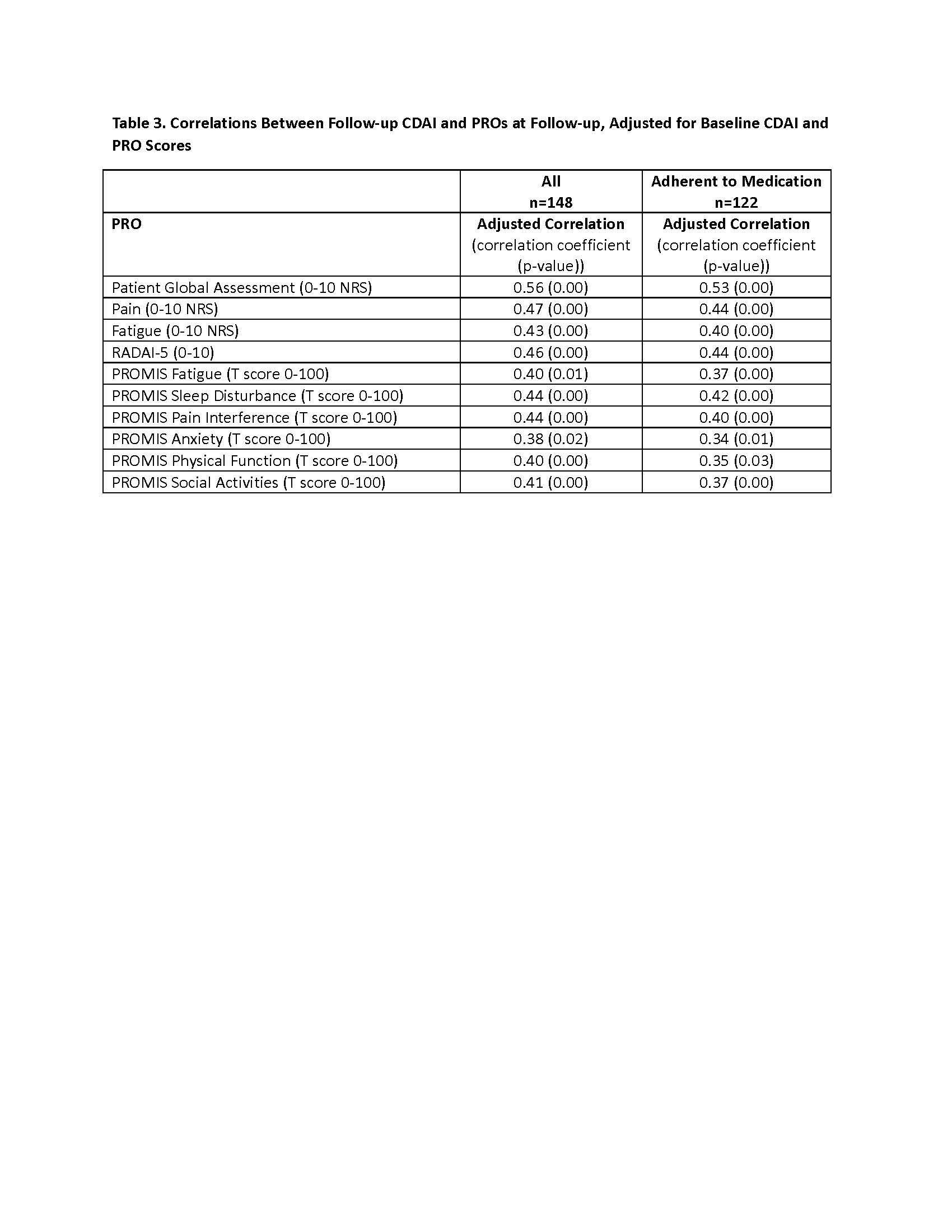
Results: Of 299 patients recruited, 240 patients (80%) successfully completed the run-in period, and 205 patients started UPA or ADA within 4 weeks and were eligible. A total of 148 patients (“All” cohort) to date have provided baseline and follow-up clinical and PRO data, and 122 (82%) remained adherent (Table 1). Mean CDAI improved 14.4 units for patients who stayed on the medication (Table 2). Correlations between follow-up CDAI and follow-up PROs ranged from 0.38 – 0.56, the strongest of which were for Patient Global Assessment NRS (R=0.56) and Pain NRS (R=0.47) (Table 3). The adjusted R2 of the overall model showed that changes in PROs explained 52% of the variability in the change in the CDAI.
Conclusion: PROs are useful for tracking RA disease activity over time outside of the clinic setting for patients initiating new treatments, even in the absence of clinician-derived measures.
All categorical data shown as n (%), all continuous data shown as mean (standard deviation [SD])
**RADAI-5 score: 0.0_1.4 for a remission-like state, 1.6_3.0 for mild disease activity, 3.2-5.4 for moderate, and 5.6_10.0 for high disease activity
PROMIS measures use a weekly referent; score of 50.0 is US population mean for all adults, 10.0 is equivalent to 1 standard deviation from the mean
PRO = patient reported outcome; NRS = Numeric rating scale; PROMIS = patient reported outcomes measurement information system; RADAI = rheumatic arthritis disease activity index
PROMIS measures use a weekly referent; score of 50.0 is US population mean for all adults, 10.0 is equivalent to 1 standard deviation from the mean
PRO = patient reported outcome; NRS = Numeric rating scale; PROMIS = patient reported outcomes measurement information system; RADAI = rheumatic arthritis disease activity index
To cite this abstract in AMA style:
Nowell W, Clinton C, Xie F, Su Y, Stradford L, Curtis D, Zueger P, Patel P, Gavigan K, Venkatachalam S, Rivera E, Curtis J. Use of Digital Health Tools to Evaluate Change in Clinical and Patient-Reported Outcomes Among Patients with Rheumatoid Arthritis Initiating Treatment with a JAKi or TNFi [abstract]. Arthritis Rheumatol. 2023; 75 (suppl 9). https://acrabstracts.org/abstract/use-of-digital-health-tools-to-evaluate-change-in-clinical-and-patient-reported-outcomes-among-patients-with-rheumatoid-arthritis-initiating-treatment-with-a-jaki-or-tnfi/. Accessed .« Back to ACR Convergence 2023
ACR Meeting Abstracts - https://acrabstracts.org/abstract/use-of-digital-health-tools-to-evaluate-change-in-clinical-and-patient-reported-outcomes-among-patients-with-rheumatoid-arthritis-initiating-treatment-with-a-jaki-or-tnfi/