Session Information
Session Type: Poster Session A
Session Time: 9:00AM-11:00AM
Background/Purpose: The NICE guideline on osteoarthritis (OA) recommends that adults aged ≥45 should be diagnosed with OA clinically, without investigations, if they have activity-related joint pain and either no morning joint-related stiffness or morning stiffness that lasts no longer than 30 minutes. While the NICE criteria are frequently used and referenced as diagnostic criteria for OA, they have not been validated. The objective of this study was to validate the NICE criteria for knee OA. This was completed within the context of a larger project focused on identifying and treating OA in people with other chronic conditions.
Methods: This was a diagnostic test study. Reporting was guided by the SIGN checklist. We recruited participants with type 2 diabetes from endocrinology clinics at three academic hospitals in Toronto, Canada. We invited individuals aged ≥45 years with and without self-reported knee pain to participate (aiming for 50% with/without). We excluded individuals with a history of inflammatory rheumatic disease. Participants first self-completed a questionnaire to identify presence of activity-related knee pain and morning joint stiffness ≤30 min (NICE criteria for OA). As history and physical exam is considered the gold standard for making an OA diagnosis, an experienced rheumatologist, blinded to the questionnaire responses, conducted a standardized clinical assessment to identify presence of knee OA (yes, no, possible). A second rheumatologist completed a subset (n=11) of assessments in duplicate for validation of the rheumatologist’s assessment. We calculated the sensitivity (sens), specificity (spec), likelihood ratio positive (LR+) and likelihood ratio negative (LR-) of the NICE criteria to detect symptomatic knee OA (yes or possible).
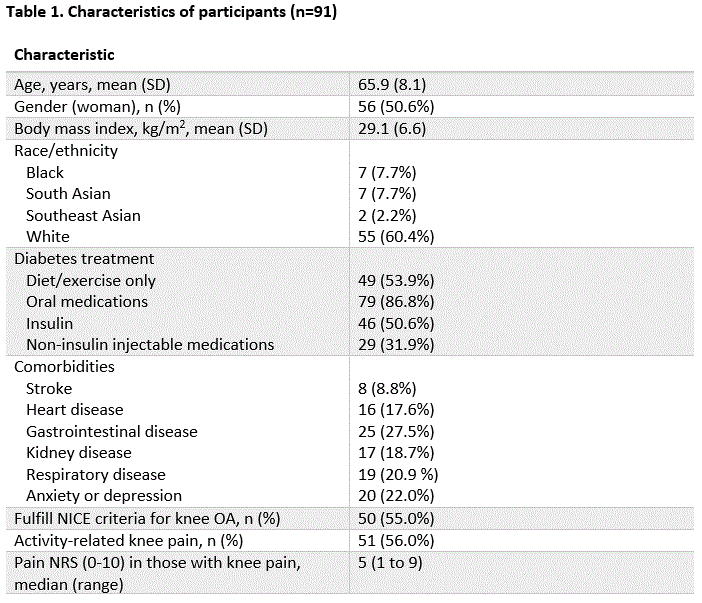
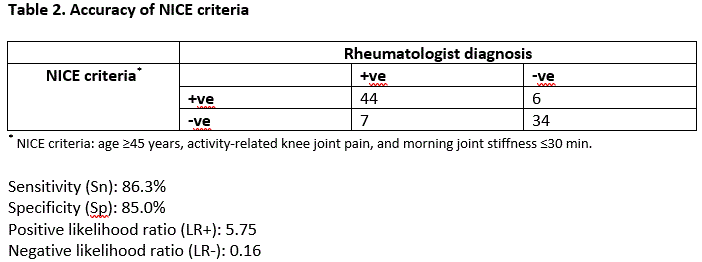
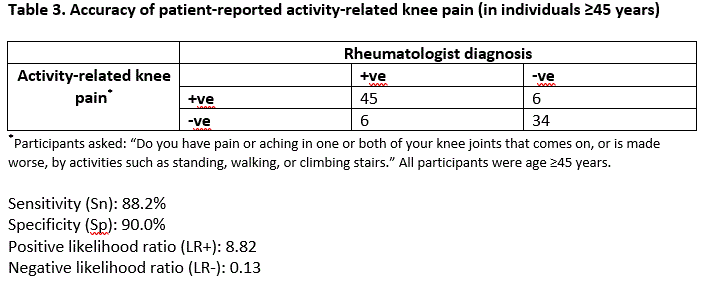
Results: Our study included 91 participants with type 2 diabetes: mean age was 65.9 (SD 8.1) years, 50.6% women, mean BMI 29.1 (SD 6.6) kg/m2 (Table 1). 50 (54.9%) fulfilled the NICE criteria with a spectrum of illness severity: median (range) pain numeric rating score (0-10) was 5 (1-9). Gold standard assessment identified 51 (56.0%) participants with symptomatic knee OA (yes: n=48, possible [suspected to be early knee OA]: n=3). The sens, spec, LR+, and LR- of NICE criteria for symptomatic knee OA were 86.3%, 85.0%, 5.75, 0.16, respectively (Table 2). Activity-related knee OA (“Do you have pain or aching in one or both of your knee joints that comes on, or is made worse, by activities such as standing, walking, or climbing stairs”) alone, without combination with morning stiffness, improved operating characteristics (88.2%, 90.0%, 8.82, 0.12) (Table 3). There was high rheumatologist inter-rater reliability for OA diagnosis (kappa = 0.84).
Conclusion: The NICE criteria have high sensitivity and specificity for detecting symptomatic knee OA. A simplified version, assessing self-reported activity-related knee pain in individuals age ≥45 years, performed slightly better and may be a preferable OA diagnostic tool. This should be validated in other settings. Given purposeful recruiting, this study cannot assess positive and negative predictive values.
To cite this abstract in AMA style:
King L, Stanaitis I, Hung V, Koppikar S, Waugh E, Lipscombe L, Hawker G. Validation of NICE Criteria for the Diagnosis of Knee Osteoarthritis [abstract]. Arthritis Rheumatol. 2023; 75 (suppl 9). https://acrabstracts.org/abstract/validation-of-nice-criteria-for-the-diagnosis-of-knee-osteoarthritis/. Accessed .« Back to ACR Convergence 2023
ACR Meeting Abstracts - https://acrabstracts.org/abstract/validation-of-nice-criteria-for-the-diagnosis-of-knee-osteoarthritis/