Session Information
Session Type: Poster Session A
Session Time: 9:00AM-11:00AM
Background/Purpose: COVID-19 vaccines have been proven to be safe in healthy populations. However, data on delayed adverse effects (AEs) in people with autoimmune diseases (AIDs), including SLE has been lacking.
Methods: COVID-19 vaccination-related AEs reported longer than 7 days post-vaccination were assessed in the “COVID-19 Vaccination in Autoimmune Diseases 2” (COVAD-2) study in patients with SLE, rheumatic autoimmune diseases other than SLE (rAIDs), non-rheumatic autoimmune diseases (nrAIRDs), and healthy controls (HC). rAIDs included connective tissue diseases, inflammatory myopathies, and inflammatory arthritis, yet not SLE, whereas nrAIDs included inflammatory bowel disease and multiple sclerosis, among others. The COVAD-2 study comprised 157 collaborators across 106 countries and was conducted between February and June 2022. An online survey captured self-reported data related to COVID-19 vaccination-associated AEs in SLE, AIDs, and HC. We compared COVID-19 vaccination-related delayed AEs between groups using multivariable binary regression adjusting for age, gender, and ethnicity.
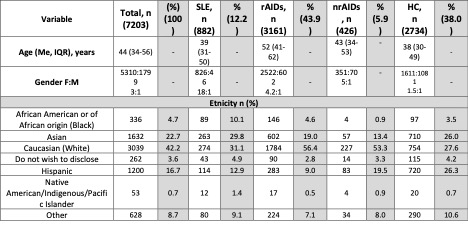
Results: Among 7203 participants, 882 (12.2%) SLE, 3161 (43.9%) rAIDs, 426 (5.9%) nrAIDs, and 2734 (38%) HC were included from a total of 10 783 respondents, with 74% female and 42.2% Caucasian (Table 1). People with SLE were young and middle-aged adults [median age 39 (31–50); rAIDs, 52 (41–62); nrAIDs 43 (34–53); HC 38 (30–49)].
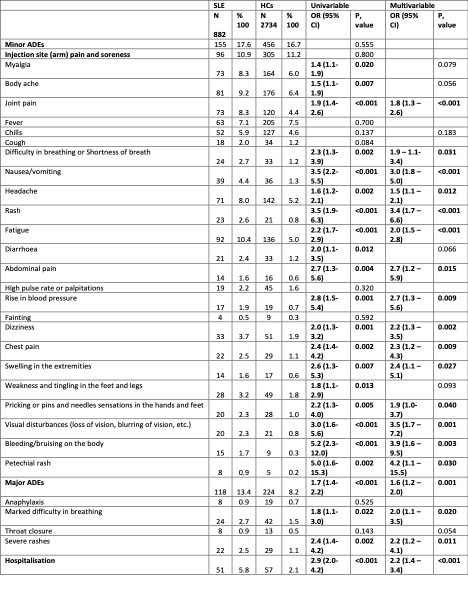
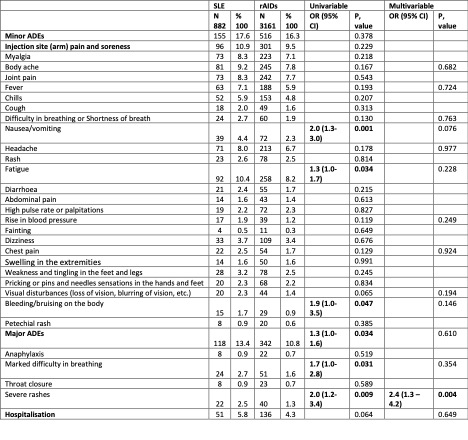
When compared to HC, people with SLE reported higher overall major [OR 1.6 (1.2–2.0)] and minor AEs (Table 2). SLE patients when compared to rAIDS reported more frequent episodes of severe rashes [OR 2.4 (1.3–4.2)], while compared to nrAIDs, SLE individuals reported an increased number of several minor AEs (joint pain [OR 2.4 (1.3–4.3)], fatigue [OR 2.0 (1.2–3.2)], body ache [OR 2.1 (1.2–3.7)]), and hospitalization [OR 2.3 (1.1–4.9)].
Vaccination with ChadOx1 nCOV-19 (Oxford/ AstraZeneca) in SLE patients led to a higher frequency in the incidence of diarrhea compared to other vaccines [OR 2.6 (1.1 – 6.2)], whereas the Moderna vaccine was associated with increased hospitalization frequencies [OR 3.0 (1.6–5.6)].
Patients with active versus non-active SLE reported similar frequencies of COVID-19 vaccine-related delayed minor AEs [17.5% versus 17.6%; OR 1.1 (0.8–1.6)]. SLE patients without autoimmune comorbidities reported fewer major and minor AEs compared to SLE patients with other concurrent rAIDs (Table 3). People with SLE and mental health disorders reported a higher frequency of severe rash [OR 3.2 (1.3–7.5)] and headache [OR 2.2 (1.3–3.6)].
Conclusion: COVID-19 vaccination is linked with increased risks of delayed AEs in patients with SLE compared to HC. Co-existence of rAIDs other than SLE yielded an increased risk for delayed AEs. By contrast, the degree of disease activity did not appear to have an impact.
To cite this abstract in AMA style:
Fathima M, Doskaliuk B, Lindblom J, Nikiphorou E, Wincup C, Saha S, Shaharir S, Katchamart W, Akarawatcharangura Goo P, Traboco L, Chen Y, Jagtap K, Lilleker J, Nune A, Pauling J, Agarwal V, Dzifa D, TORO GUTIERREZ C, CABALLERO C, Chinoy H, Aggarwal R, Agarwal V, Gupta L, Parodis I. COVID-19 Vaccination-related Delayed Adverse Events Among Patients with Systemic Lupus Erythematosus: Results from the COVAD Study [abstract]. Arthritis Rheumatol. 2023; 75 (suppl 9). https://acrabstracts.org/abstract/covid-19-vaccination-related-delayed-adverse-events-among-patients-with-systemic-lupus-erythematosus-results-from-the-covad-study/. Accessed .« Back to ACR Convergence 2023
ACR Meeting Abstracts - https://acrabstracts.org/abstract/covid-19-vaccination-related-delayed-adverse-events-among-patients-with-systemic-lupus-erythematosus-results-from-the-covad-study/