Session Information
Session Type: Poster Session A
Session Time: 6:00PM-7:00PM
Background/Purpose: Children receiving immunosuppressive therapies (IST) have a higher risk of hospitalization from COVID-19. COVID-19 vaccines significantly reduce the likelihood of severe disease or death. Early studies demonstrate safety and immunogenicity of COVID-19 vaccines in children with rheumatic diseases. Yet, as of December 2022, 63% of children 6 months to 17 years of age remain unvaccinated against COVID-19. The CARRA Vaccination Working Group (WG) surveyed pediatric rheumatologists to evaluate current COVID-19 vaccination practices, vaccine hesitancy and barriers.
Methods: The CARRA Vaccination WG developed and distributed a survey to CARRA member healthcare providers from March-May 2022. The survey included questions about COVID-19 vaccination practices and provider report of parent perceptions about COVID-19 vaccination for their children. Results were collected via RedCap and responses were analyzed.
Results: The survey was completed by 219 members, with 74% pediatric rheumatologists and 21% fellows. The majority (98%) opinion was that disease flares after COVID-19 vaccination would be mild and/or rare. Provider concerns about vaccine-associated adverse events (AEs) included risk of myocarditis (76%), new autoimmune conditions (29%), and thrombosis (22%). These AEs were ranked as low risk, with 98% of providers recommending COVID-19 vaccines for their patients. Concerns of decreased vaccine efficacy were reported by 59%, particularly among patients receiving the following IST: rituximab (100%), systemic corticosteroids (86%), mycophenolate mofetil (59%), and JAK-inhibitors (46%). IST was temporarily modified for vaccination by 88% of providers, mostly based on ACR guidelines. Most providers (82%) did not routinely check post-vaccination serology and the remainder did so primarily for research purposes. Notably, 98% of providers reported encountering parents declining COVID-19 vaccination, with 75% reporting hesitancy in >10% of patients. In contrast, for routine vaccines, only 25% reported hesitancy in >10% of patients. Reported reasons for parent COVID-19 vaccine hesitancy were concerns about side effects, lack of long-term safety data, prior COVID-19 infection, and vaccine misinformation such as risk of infertility, genetic changes, or vaccination leading to COVID-19 infection.
Conclusion: Our survey showed discordance between provider and perceived parent opinions regarding COVID-19 vaccines for children with rheumatic diseases. Concerns about vaccine efficacy and AEs, including vaccine-associated myocarditis, did not reduce provider recommendations for COVID-19 vaccination. In contrast, parent concern of vaccine side effects, lack of long-term safety data, and misinformation reduced the likelihood of parents agreeing to vaccinate their child. These results highlight discrepancies between providers and parents of children with rheumatic disease in balancing the risks and benefits of COVID-19 vaccination. Limitations of the study are that providers rather than parents provided the reasons for vaccine hesitancy. Our study underscores the need to survey parents directly and include parents in the design phase of COVID-19 vaccination studies for children.
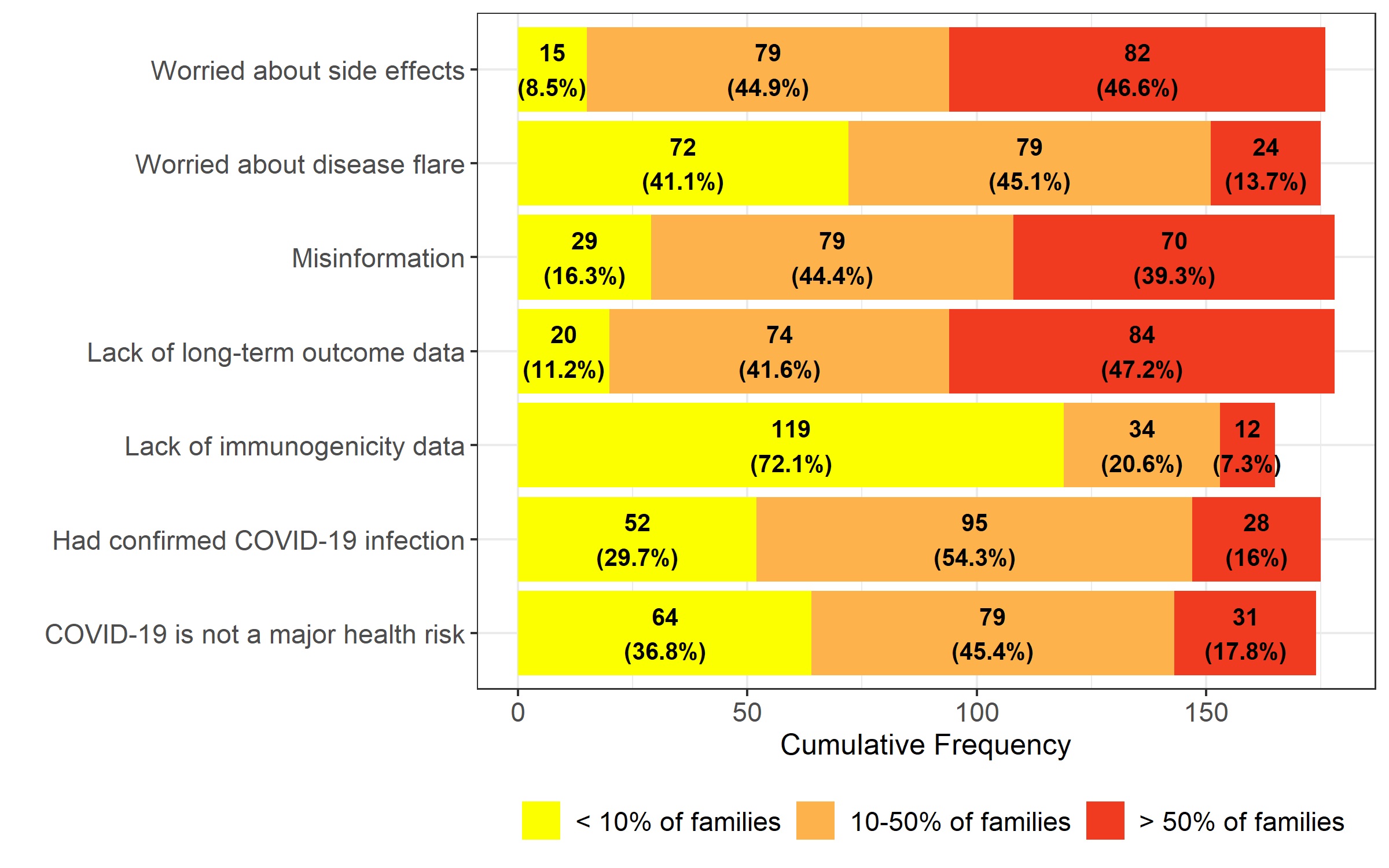 Reasons for patients/ families declining COVID_19 vaccination per rheumatology providers
Reasons for patients/ families declining COVID_19 vaccination per rheumatology providers
To cite this abstract in AMA style:
Rutstein B, Heshin Bekenstein M, Schletzbaum M, Singer N, Sadun R, Kohlheim M, Del Gaizo V, Wise K, Nikahd M, Brock G, Ardura M, Sivaraman V, CARRA Investigators F. COVID-19 Vaccination in Children with Rheumatic Diseases: Results of a CARRA-wide Survey [abstract]. Arthritis Rheumatol. 2023; 75 (suppl 4). https://acrabstracts.org/abstract/covid-19-vaccination-in-children-with-rheumatic-diseases-results-of-a-carra-wide-survey/. Accessed .« Back to 2023 Pediatric Rheumatology Symposium
ACR Meeting Abstracts - https://acrabstracts.org/abstract/covid-19-vaccination-in-children-with-rheumatic-diseases-results-of-a-carra-wide-survey/
