Session Information
Session Type: Poster Session D
Session Time: 1:00PM-3:00PM
Background/Purpose: Immunosuppression in Takayasu Arteritis (TA) reduces the risk of arterial damage and disease progression. However, long-term use of glucocorticoids (GC) and other immunosuppressants carries risk, particularly as patients age. GC tapering protocols vary between centres and there are no current guidelines on the safety of treatment cessation due to a lack of data. This study describes the cessation of treatment in a large single centre cohort and evaluates the baseline, clinical and treatment parameters associated with successful treatment stop.
Methods: The Imperial College Takayasu Arteritis Cohort (UK) was reviewed retrospectively; 158 patients with median follow-up 8.4 [5-13.6]yrs. Patients with data available within 1 year of the audit date (March 31st 2021) were analysed. Treatment was defined as >6 months of GC, DMARD or biologic medication and cessation as stopping all treatment for >1 year. Cessation safety was assessed by serial angiography and NIH disease activity scoring. Baseline demographic, clinical and treatment parameters as well as initial treatment responses were compared for cessation and non-cessation cases. Data are median [IQR], comparisons are non-parametric for continuous variables.
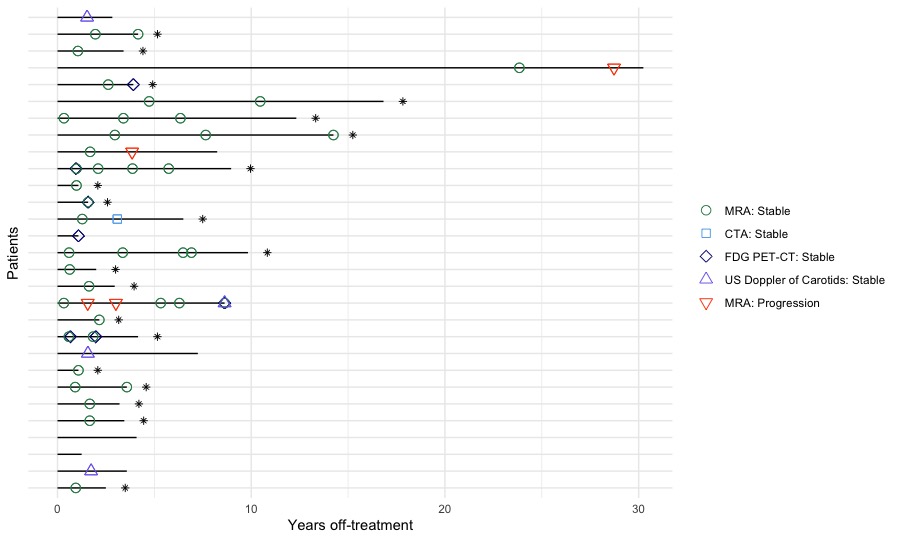
Results: Within 158 TA cases; 129 (82%) required treatment (18% presented with burnt-out disease). 107 cases (83%) remained in follow-up care. Treatment was ceased in 29 cases (27.1%), time off-treatment was 3.6 [2.5-8.2]yrs. A single case of post-cessation disease relapse requiring treatment restart was identified but did not meet cessation criteria ( < 1 yr off-treatment). For 25 (86%) cessation cases, treatment withdrawal was due to sustained disease inactivity while in 4 cases withdrawal was accelerated by infection, non-adherence and tolerance. Activity scoring of cessation cases confirmed disease quiescence. Serial angiography (aorta MRI or CT angiogram) confirmed no arterial progression in 20 cases (Fig. 1). Two cases with findings indicative of progression were later diagnosed as atherosclerosis and one case had aortic dilatation unaccompanied by systemic features.
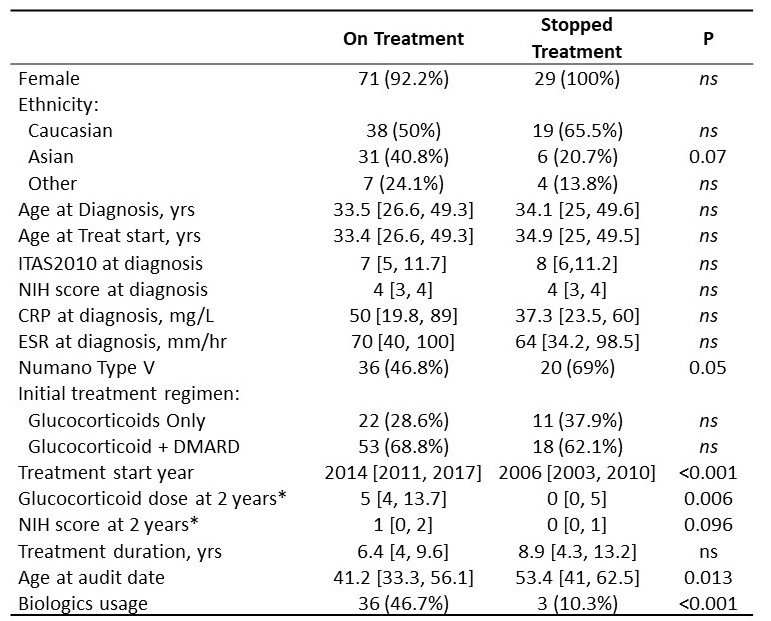
Baseline demographic and disease severity parameters were similar in cessation and non-cessation patients (Table 1). Cessation cases had lower GC doses after 2 yrs treatment and a trend towards lower disease activity, suggesting better responses. Consistent with this, biologic administration (reserved for refractory disease) was markedly lower in the cessation group. Finally, cessation cases had earlier treatment start years, were older at the audit date and had an increased proportion of Numano Type V classification.
Conclusion: In TA, complete treatment cessation is feasible and safe in a proportion of cases. Initial treatment response, treatment duration and Numano type V arterial involvement are potential predictors of successful treatment cessation and may aid future stratification strategies after validation.
Black lines represent a single patient, length of line is patient’s time off-treatment. Asterisk (*) indicates patient with conclusive stable angiography. MRA, MRI based whole aorta angiography; CTA, CT based whole aorta angiography, FDG-PET-CT, fluorodeoxyglucose positron emission tomography; US, ultrasound.
* available data for 61 and 11 On Treatment and Stopped Treatment cases respectively
To cite this abstract in AMA style:
Maughan R, Porter A, Dahanayake C, Ianonne C, Alapat R, Pericleous C, Youngstein T, Mason J. Evaluating the Safety and Factors Associated with Treatment Cessation in Takayasu Arteritis [abstract]. Arthritis Rheumatol. 2022; 74 (suppl 9). https://acrabstracts.org/abstract/evaluating-the-safety-and-factors-associated-with-treatment-cessation-in-takayasu-arteritis/. Accessed .« Back to ACR Convergence 2022
ACR Meeting Abstracts - https://acrabstracts.org/abstract/evaluating-the-safety-and-factors-associated-with-treatment-cessation-in-takayasu-arteritis/