Session Information
Session Type: Abstract Session
Session Time: 3:00PM-4:30PM
Background/Purpose: Baricitinib is a JAK1/2 selective inhibitor approved for the treatment of rheumatoid arthritis. Juvenile idiopathic arthritis (JIA) is a group of diseases characterized by immune mediated chronic arthritis which often requires treatment with conventional synthetic or biologic disease-modifying antirheumatic drugs (cs or b-DMARDs). To investigate baricitinib efficacy and safety in pediatric patients with JIA and an inadequate response to cs or b-DMARDs.
Methods: This Phase 3 multicenter, double-blind, withdrawal, efficacy, and safety study, enrolled patients (pts) age 2 to < 18 years with extended oligo- or poly-articular JIA, ERA, or JPsA, per ILAR criteria, and an inadequate response to ≥1 cs and/or b-DMARDs (NCT03773978). There were 3 periods: a 2-week (wk) pharmacokinetic/safety assessment (PKS), a 12-wk open-label lead-in (OLLI), and an up-to 32-wk double-blind withdrawal (DBW). Dosage and safety were confirmed in the PKS and then pts, including those from the PKS, enrolled in the OLLI, receiving age-based, oral, once daily doses of baricitinib. Pts with a JIA-ACR30 response at wk12, end of OLLI, entered the DBW to be randomized 1:1 to continued baricitinib or newly started placebo (PBO) and remained until flare or up to wk32. Primary endpoint was time to flare during the DBW. Secondary endpoints included JIA-ACR30/50/70/90 response rates at wk12, and proportion of pts with a flare during the DBW. Survival curves were estimated using the Kaplan-Meier method.
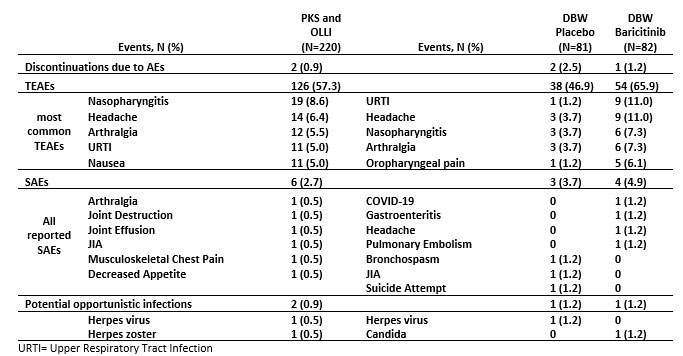
Results: Of 220 pts enrolled, 29 participated in the PKS, 219 entered the OLLI, and 163 entered the DBW. The JIA-ACR30/50/70/90 response at wk12 was 76.3%/63.5%/46.1%/20.1%, respectively. During the DBW, time of flare was significantly shorter with PBO vs baricitinib (hazard ratio 0.24 [95% CI 0.13,0.45], p< 0.001; Figure 1). The proportion of pts with a flare during the DBW was significantly lower for baricitinib vs PBO (14 (17.1%) vs. 41 (50.6%), p< 0.001). In the PKS and OLLI periods, 126 (57.3%) pts reported ≥1 treatment emergent adverse event (TEAE), while 6 (2.7%) reported ≥1 serious adverse event (SAE); Table 1. In the DBW, 38 (46.9%) and 54 (65.9%) pts reported ≥1 TEAE for PBO and baricitinib, respectively, whereas those with ≥1 SAE were 3 (3.7%) and 4 (4.9%). The mean wks of exposure was higher in the baricitinib vs PBO group during DBW (26.34 vs 18.91) due to study design. There were no deaths, cardiovascular events or uveitis and 1 case of herpes zoster.
Conclusion: Baricitinib significantly reduced time to and frequency of JIA flares in pts with JIA versus PBO, and improved JIA-ACR scores in the majority of pts within 12wks. Safety findings were consistent with the known safety profile in adult rheumatoid arthritis indications. These findings support baricitinib as a treatment for signs and symptoms of JIA with an inadequate response to cs or b-DMARDs.
References:
1. Giannini EH, et. al. Preliminary definition of improvement in juvenile arthritis. Arthritis Rheum 1997; 40: 1202-1209.
2. Brunner HI, et. al. Preliminary definition of disease flare in juvenile rheumatoid arthritis. J Rheumatol 2002; 29(5):1058-64.
To cite this abstract in AMA style:
Ramanan A, Quartier Dit Maire P, Okamoto N, Meszaros G, Araujo J, Wang Z, Liao R, Crowe B, Zhang X, Decker R, Keller S, Brunner H, Ruperto N. Baricitinib in Juvenile Idiopathic Arthritis: A Phase 3, Double-Blind, Placebo-Controlled, Withdrawal, Efficacy and Safety Study [abstract]. Arthritis Rheumatol. 2022; 74 (suppl 9). https://acrabstracts.org/abstract/baricitinib-in-juvenile-idiopathic-arthritis-a-phase-3-double-blind-placebo-controlled-withdrawal-efficacy-and-safety-study/. Accessed .« Back to ACR Convergence 2022
ACR Meeting Abstracts - https://acrabstracts.org/abstract/baricitinib-in-juvenile-idiopathic-arthritis-a-phase-3-double-blind-placebo-controlled-withdrawal-efficacy-and-safety-study/