Session Information
Session Type: Abstract Session
Session Time: 3:00PM-4:30PM
Background/Purpose: Many patients with autoimmune and inflammatory conditions discontinue biologics and other immunomodulatory medications prematurely, but many fail to even start (primary non-adherence). We examined primary non-adherence in a national rheumatology EHR data warehouse linked to pharmacy claims data.
Methods: We identified patients with rheumatoid arthritis, axial spondyloarthritis, psoriatic arthritis or psoriasis who newly initiated TNFi and non-TNFi biologics, and conventional and targeted synthetic DMARDs in the Illumination Health Real-World Evidence Platform linked to fee-for-service Medicare or commercial health plan claims data. Patients with new prescriptions identified in the EHR data were compared to the gold standard of actual fills or administrations in linked claims data at 3 and 6 months. We examined factors associated with non-adherence using KM curves and estimated hazard ratios (HRs) from Cox proportional hazards models, comparing each medication class to intravenous (IV) and subcutaneous (SC) TNFi.
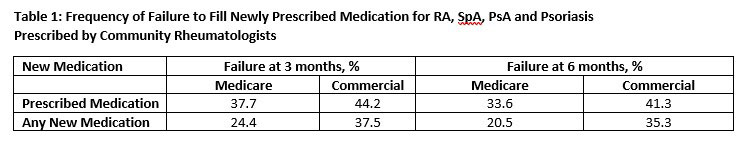
Results: In Medicare, 1239 patients were prescribed 1577 immunomodulatory medications, with mean (SD) age 69 (10) years. Similarly, 258 commercially-insured patients initiated 317 medications, mean(SD) age 64(13) years. Approximately 60% had RA. The most common newly prescribed medications were csDMARDs(Medicare: 50%; commercial: 43%), TNFi (Medicare:18%; commercial: 23%), non-TNFi biologics (Medicare:16%; commercial: 17%), and Janus kinase inhibitors [JAKi] (Medicare: 16%; commercial: 15%). Following the first prescription in the EHR data and within the next 3 or 6 months, patients frequently did not fill the prescribed medication (Table 1). Medicare patients were most likely to fill csDMARDs and IV biologics (Figure).
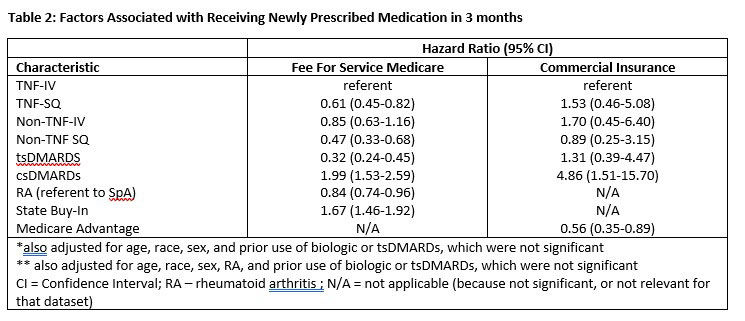
After multivariable adjustment, and referent to TNFi-IV, medication class was significantly associated with primary non-adherence (Table 2). In Medicare data, IV TNFi drugs and csDMARDs were most likely to be filled in Medicare data, whereas for commercially insured patients, subcutaneous TNFi and csDMARDs were most likely to be filled.
Conclusion: Between 20 and 44% of newly prescribed biologic and immunomodulatory medications for RA and SpA were not filled within 6 months. Approaches to quickly detect this non-adherence shortly after prescribing are needed to maximize the benefits of early initiation of these important therapies.
To cite this abstract in AMA style:
Curtis J, Su Y, Clinton C, Stewart P, England B, Buekelman T, Xie F. Primary Non-adherence to Biologics and Immunomodulatory Therapies for Rheumatoid Arthritis, Psoriatic Arthritis, and Spondyloarthritis Using Linked EHR and Pharmacy Claims Data [abstract]. Arthritis Rheumatol. 2022; 74 (suppl 9). https://acrabstracts.org/abstract/primary-non-adherence-to-biologics-and-immunomodulatory-therapies-for-rheumatoid-arthritis-psoriatic-arthritis-and-spondyloarthritis-using-linked-ehr-and-pharmacy-claims-data/. Accessed .« Back to ACR Convergence 2022
ACR Meeting Abstracts - https://acrabstracts.org/abstract/primary-non-adherence-to-biologics-and-immunomodulatory-therapies-for-rheumatoid-arthritis-psoriatic-arthritis-and-spondyloarthritis-using-linked-ehr-and-pharmacy-claims-data/