Session Information
Date: Monday, November 14, 2022
Title: SLE – Diagnosis, Manifestations, and Outcomes Poster III: Outcomes
Session Type: Poster Session D
Session Time: 1:00PM-3:00PM
Background/Purpose: The Systemic Lupus Erythematosus Responder Index 4 (SRI-4) and the British Isles Lupus Assessment Group (BILAG)-based Composite Lupus Assessment (BICLA) are currently the most common primary endpoints in systemic lupus erythematosus (SLE) randomized-controlled trials (RCTs). However, discordance between SRI-4 and BICLA effect sizes in studies has engendered skepticism about the utility of these composite indices. In this post-hoc analysis of data from six RCTs of non-renal SLE, we examined concordance and discordance in the SRI-4 and BICLA outcome measures among placebo recipients, and explored reasons for SRI-4/BICLA nonresponse.
Methods: Placebo data from SLE RCTs of 24- or 52-week duration that collected the Systemic Lupus Erythematosus Disease Activity Index (SLEDAI) and BILAG 2004 Index were included. Participants in each study were classified as either a responder or nonresponder by the SRI-4 and BICLA composite indices, and frequencies of concordance and discordance between the SRI-4 and BICLA were determined. Overall agreement between SRI-4 and BICLA was estimated by Cohen’s kappa. For each participant, the leading reason for nonresponse by the SRI-4 and BICLA was identified by the following hierarchical order: concomitant medication violation; study withdrawal; lack of improvement by SLEDAI or BILAG, respectively; worsening disease by BILAG; worsening disease by SLEDAI (as a criterion for BICLA nonresponse); and worsening physician global assessment.
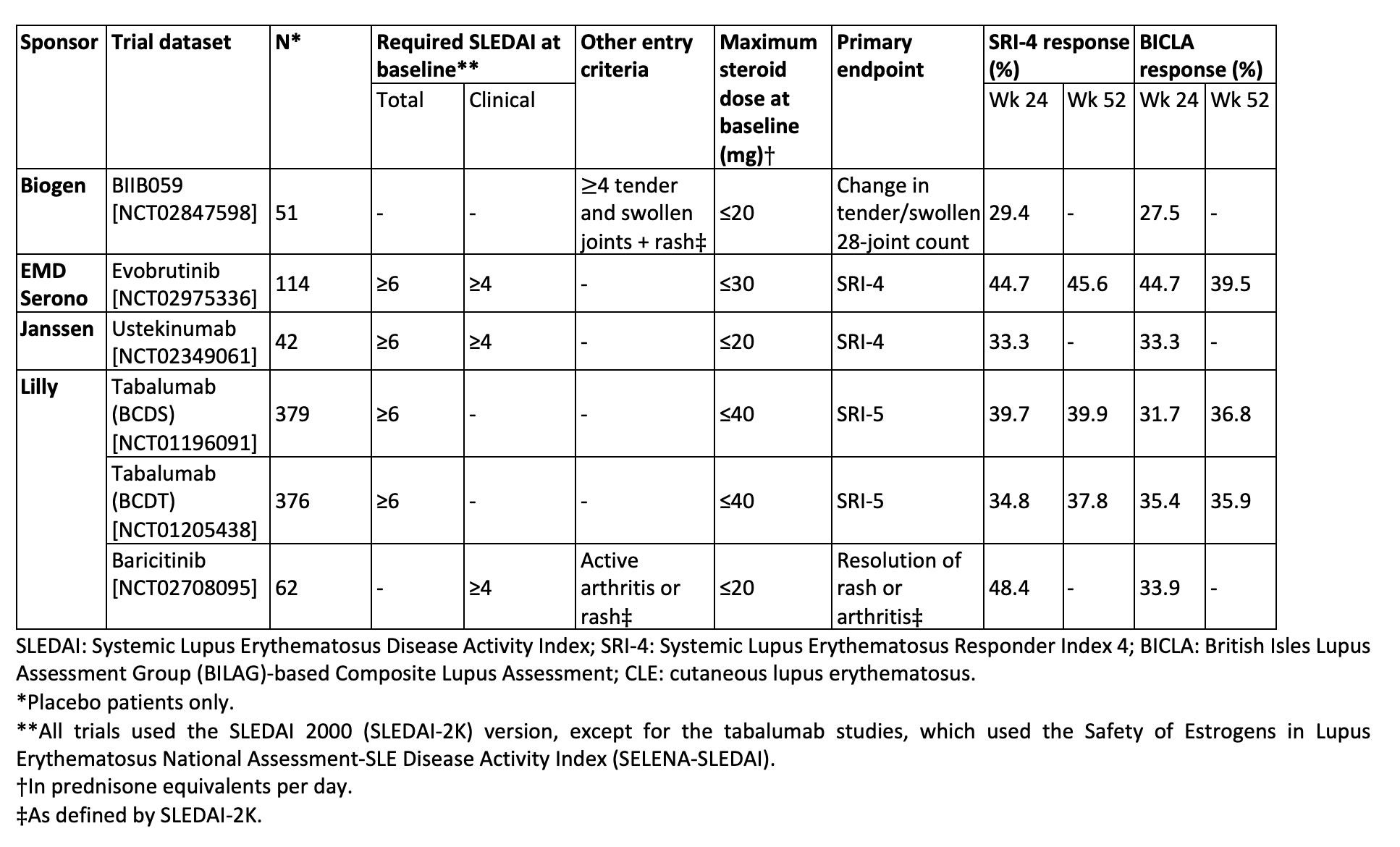
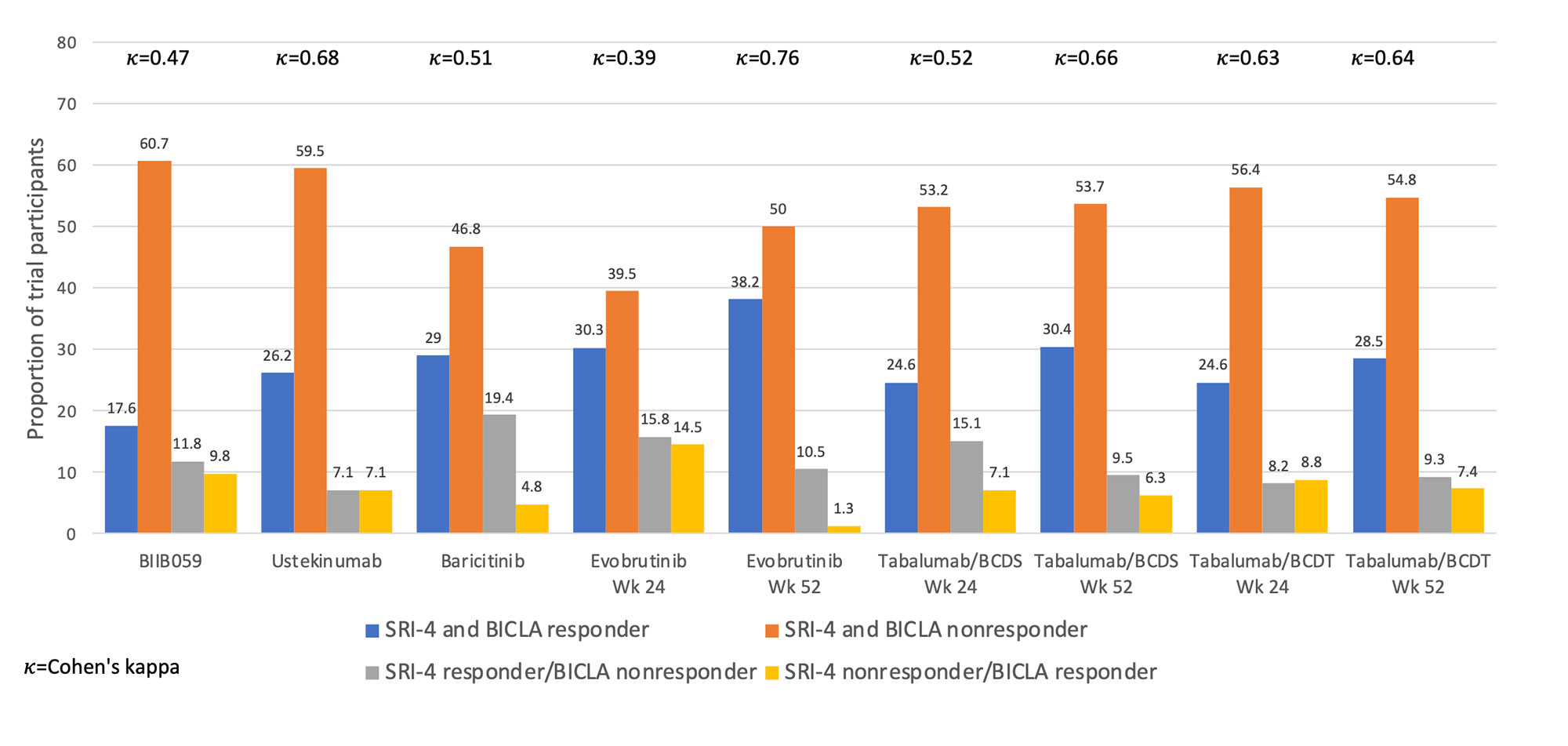
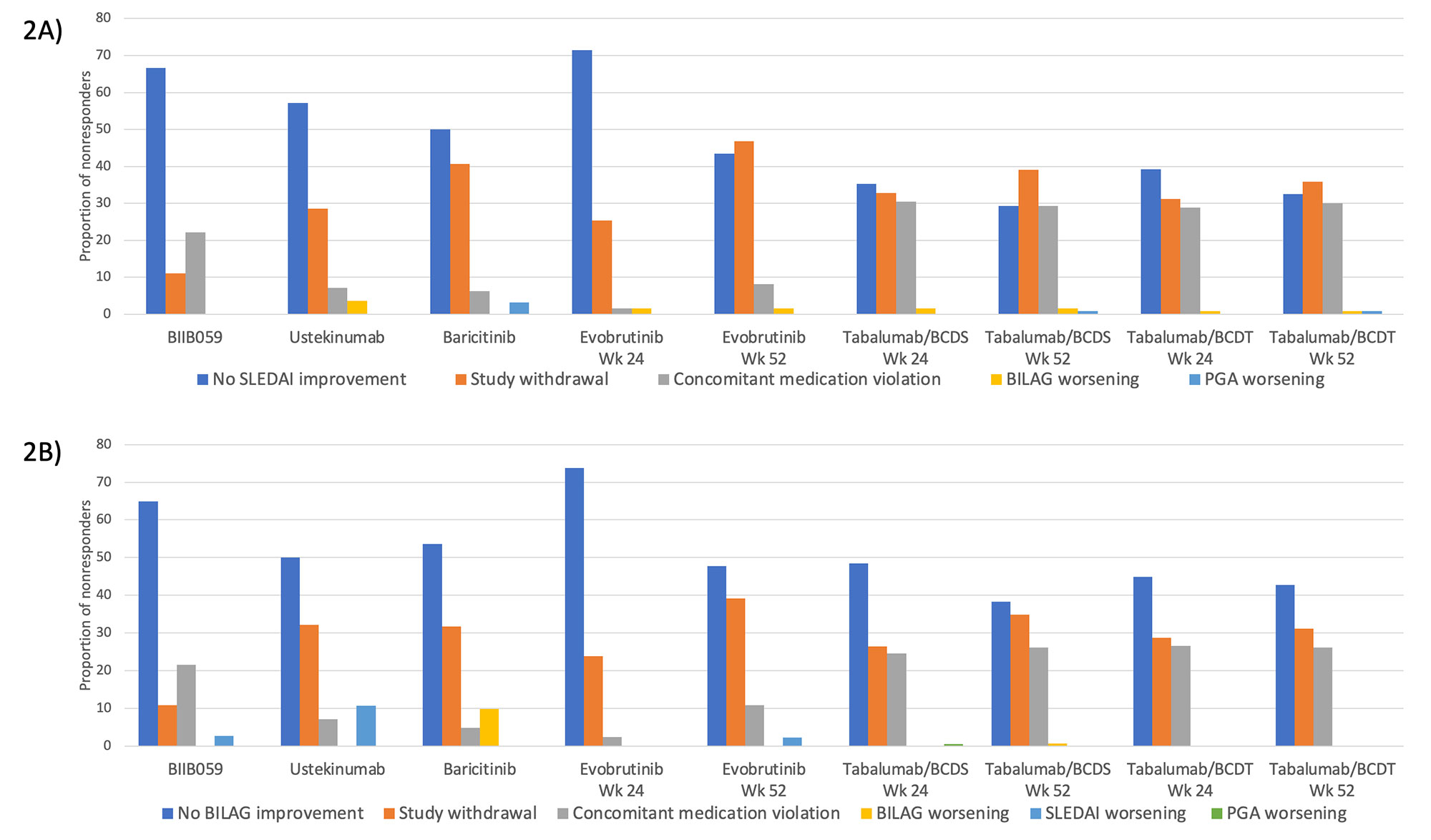
Results: Five of six RCTs had a requirement for minimum total or clinical SLEDAI score at entry (Table 1). Four trials had primary endpoints of SRI-4 or SRI-5, and all had steroid dose limits at baseline. Placebo response rates ranged from 29-48% with SRI-4 as the outcome and 28-45% with BICLA as the outcome. As shown in Figure 1, SRI-4 and BICLA concordance varied by clinical trial with a Cohen’s kappa ranging from 0.39-0.76. Within trials that provided data at both timepoints, agreement of SRI-4 and BICLA was higher at 52 weeks than at 24 weeks. The three most common reasons for SRI-4 and BICLA nonresponse were lack of SLEDAI or BILAG improvement, respectively, study withdrawal, or concomitant medication violation (Figure 2).
Conclusion: Discordance between the SRI-4 and BICLA varied by clinical trial, ranging from 11.8-30.3%, with SRI-4 classifying more responders than the BICLA. Nonresponse to SRI-4 and BICLA was largely due to lack of clinical improvement by the SLEDAI or BILAG, respectively, study withdrawal, or concomitant medication violation. The remaining components of the SRI-4 and BICLA provided minimal impact to nonresponder frequencies. Further research will identify the specific factors associated with SRI-4 and BICLA discordance.
To cite this abstract in AMA style:
Aguirre A, Kim M, Goteti K, Li Y, Kao A, Franchimont N, Kong G, Barbey C, Zuraw Q, Gordon R, Manner D, Silk M, Staeva T, Nguyen H, Furie R, Linnik M, Dall'Era M. SRI-4 and BICLA: How Well Do They Agree Across Trials of Active Systemic Lupus Erythematosus? [abstract]. Arthritis Rheumatol. 2022; 74 (suppl 9). https://acrabstracts.org/abstract/sri-4-and-bicla-how-well-do-they-agree-across-trials-of-active-systemic-lupus-erythematosus/. Accessed .« Back to ACR Convergence 2022
ACR Meeting Abstracts - https://acrabstracts.org/abstract/sri-4-and-bicla-how-well-do-they-agree-across-trials-of-active-systemic-lupus-erythematosus/