Session Information
Date: Monday, November 14, 2022
Title: Plenary III
Session Type: Plenary Session
Session Time: 11:30AM-1:00PM
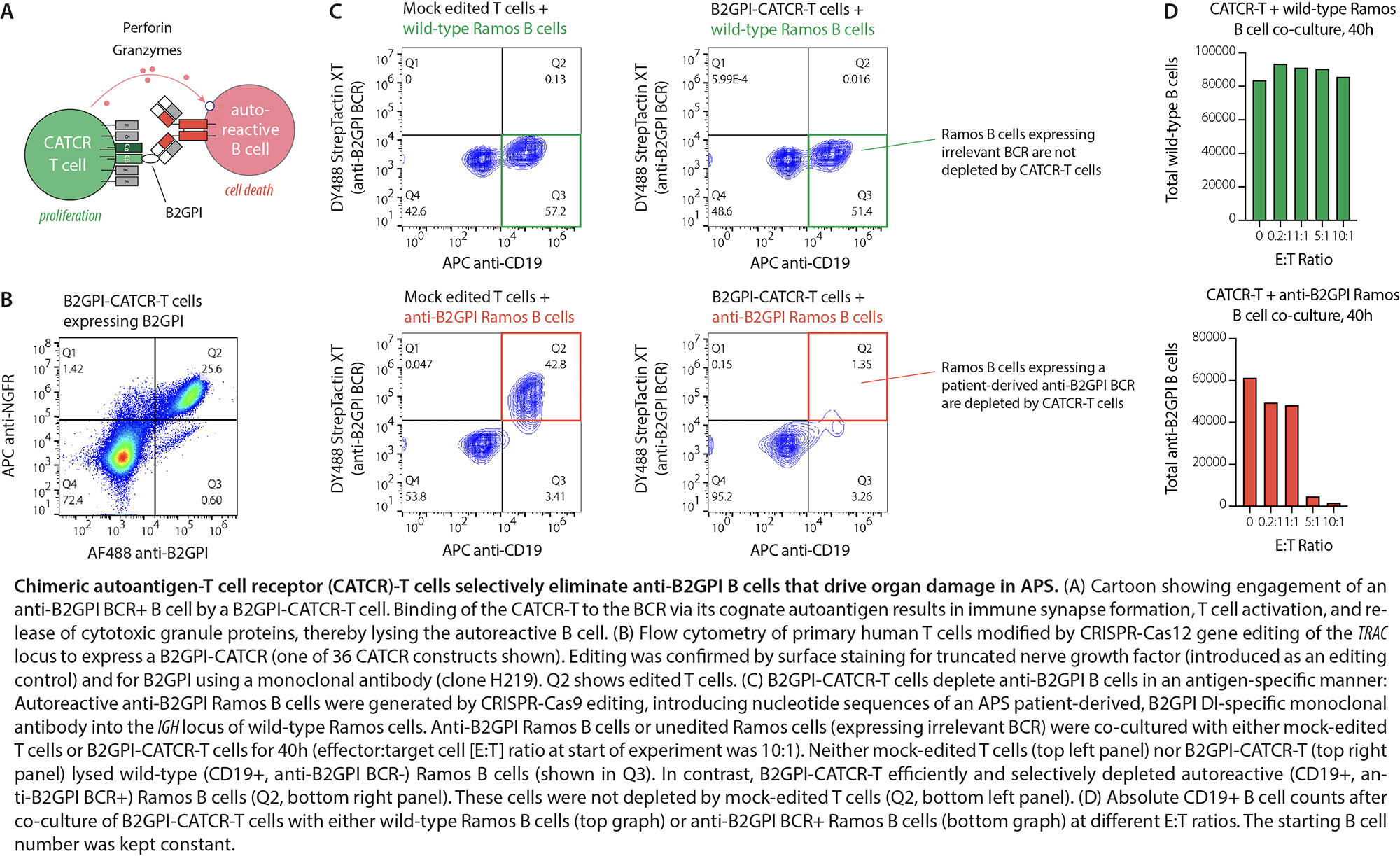
Background/Purpose: Chimeric antigen receptor (CAR)-T cell therapies have revolutionized the treatment of cancer and can be curative. CD19-targeted CAR-T cells hold promise for the treatment of refractory autoimmune diseases, but therapies that indiscriminately deplete all B cells are limited by infection risk. In contrast, precision immunotherapies that selectively eliminate autoreactive B cells, while maintaining the normal B cell pool, have the potential to treat autoimmunity without impairing protective immunity. Antiphospholipid syndrome (APS) is characterized by autoantibodies against beta-2-glycoprotein I (B2GPI) domain I (DI) which are pathogenic and promote thrombosis and fetal loss. We therefore aimed to develop an autoantigen-specific T-cell therapy targeting anti-B2GPI B cells for the treatment of APS.
Methods: We employed a CRISPR-Cas9/12a-based genome-editing platform to incorporate APS-relevant autoantigen into the T cell receptor (TCR)-CD3 complex of primary human T cells. Homology-directed repair template encoding partial or full nucleotide sequences of B2GPI and Cas-gRNA ribonucleoprotein complexes targeting CD3G, CD3E, or TRAC were transfected into activated T cells. CATCR expression was quantified by flow cytometry. Ramos B cells were CRISPR-Cas9-edited to replace their native BCR with monoclonal anti-B2GPI DI BCRs cloned from patients with APS. The potency and specificity of 36 B2GPI-CATCR-T cells against different anti-B2GPI or wild-type Ramos B cells was interrogated in co-culture, and cytotoxicity was quantified by flow cytometry, live-cell imaging, and cytokine assays.
Results: We hypothesized that T cells can be redirected to selectively kill anti-B2GPI B cells in APS. To this end, T cells were engineered to express chimeric autoantigen-TCRs (CATCRs) which confer the ability to bind autoantigen-specific B-cell receptors (BCRs), thereby inducing immune synapse formation and killing of autoreactive B cells via the perforin–granzyme effector pathway (Fig.1A). B2GPI-CATCR-T cells, expressing the APS autoantigen B2GPI as part of CD3-gamma, CD3-epsilon, or the TCR-beta/alpha chains, were successfully generated from primary T cells, as confirmed by flow cytometry (Fig.1B). In co-culture of T cells with autoreactive Ramos B cells (expressing pathogenic anti-B2GPI BCRs/autoantibody) or unedited Ramos cells (expressing irrelevant BCR), B2GPI-CATCR-T cells -but not control T cells- efficiently and selectively eliminated anti-B2GPI DI B cells. In contrast, B2GPI-CATCR-T cells did not deplete B cells expressing irrelevant BCR (Fig.1C). Target cell killing was observed at physiologically relevant effector:target cell ratios (Fig.1D).
Conclusion: We describe a precision cellular immunotherapy approach for the autoantigen-specific depletion of autoreactive B cells in autoimmune diseases, which we here first applied to APS. B2GPI-CATCR-T cells selectively eliminated anti-B2GPI DI B cells—pathogenic drivers of thrombosis and fetal loss in APS—in a dose-dependent manner, providing an opportunity to treat and prevent APS. Beyond APS, these autoantigen-specific immunotherapies promise a future of treating autoimmunity without increasing the risk of infection.
To cite this abstract in AMA style:
Mog B, Shaw E, Hwang M, Pearlman A, DiNapoli S, Paul S, Bettegowda C, Papadopoulos N, Gabelli S, Petri M, Rosen A, Zhou S, Kinzler K, Vogelstein B, Konig M. Chimeric Autoantigen-T Cell Receptor (CATCR)-T Cell Therapies to Selectively Target Autoreactive B Cells [abstract]. Arthritis Rheumatol. 2022; 74 (suppl 9). https://acrabstracts.org/abstract/chimeric-autoantigen-t-cell-receptor-catcr-t-cell-therapies-to-selectively-target-autoreactive-b-cells/. Accessed .« Back to ACR Convergence 2022
ACR Meeting Abstracts - https://acrabstracts.org/abstract/chimeric-autoantigen-t-cell-receptor-catcr-t-cell-therapies-to-selectively-target-autoreactive-b-cells/