Session Information
Session Type: Abstract Submissions (ACR)
Background/Purpose:
In the RAPID2 randomized controlled trial (RCT; NCT00160602), certolizumab pegol (CZP) +MTX every 2 weeks (Q2W) improved signs and symptoms of rheumatoid arthritis (RA) over 24 weeks (wks).1 Previous results demonstrated long-term safety and efficacy of CZP+MTX over 3 yrs in RAPID2 open-label extension (OLE).2 We present the final report on long-term safety and efficacy of CZP+MTX over 5 yrs.
Methods:
Eligible patients (pts) from RAPID2 RCT were treated in OLE (NCT00160641) with CZP 400mg Q2W, reduced to 200mg Q2W after ≥6 months, +MTX. Combined safety data from RCT and OLE are reported for all pts treated with ≥1 dose of CZP (N=612). AEs and SAEs were assessed at each visit following first dose of CZP. DAS28(ESR), HAQ-DI and ACR20/50/70 are presented to Wk232 for CZP Completers (pts who completed RCT and enrolled onto OLE [N=342]) and CZP ITT population (all pts randomized to CZP in RCT [N=492]). Change from baseline in modified Total Sharp Score (mTSS) and % of pts with radiographic non-progression (mTSS change from RCT baseline ≤0.5) are reported to Wk128 for CZP Completers. Dose reduction efficacy data is presented for all Wk24 CZP Completers who received CZP 400mg Q2W +MTX for ≥6 months in OLE, following which the CZP dose was reduced to 200mg Q2W over 132 wks of CZP exposure. Modified non-responder imputation (mNRI) was used for ACR responses; LOCF for continuous efficacy measures; mTSS data imputed by linear extrapolation.
Results:
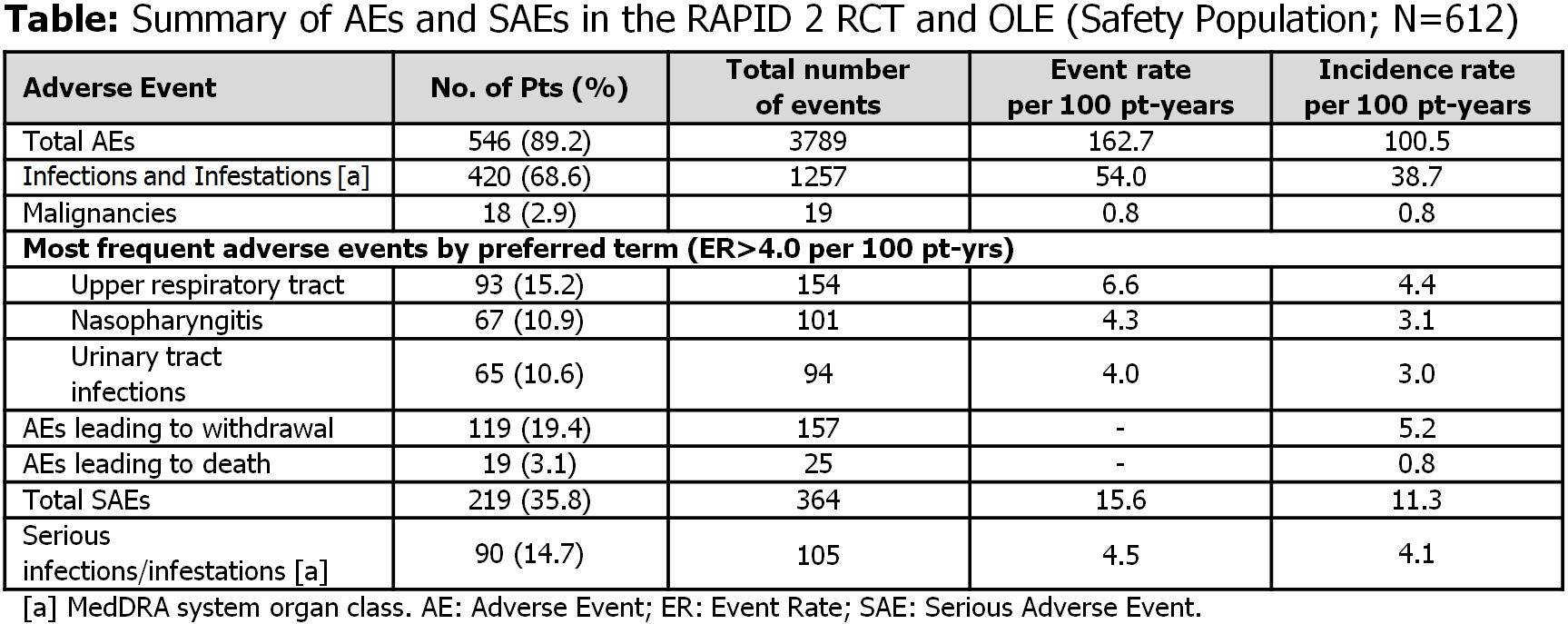
Of 492 pts treated with CZP+MTX, 355 (72%) completed the RCT and 342 entered OLE, of which 215 remained after 232 wks from RCT baseline. Safety profile was consistent with previous reports. Most frequent AEs (preferred terms) are reported (Table). 19 pts (3.1%) died (IR=0.82) (including 5 malignancies, 4 cardiac disorders, 4 nervous system disorders, 4 injuries). No new safety signals were identified. Clinical improvements from RCT were maintained to Wk232 in CZP Completers and ITT Population, respectively: mean DAS28(ESR), 3.7 and 3.9; mean HAQ-DI, 0.96 and 1.06; ACR20/50/70, 68.4%/47.1%/25.1% and 65.9%/45.4%/24.2%. Radiographic progression in CZP-treated pts was minimal (mean mTSS change from RCT baseline to Wk24: 0.62, from RCT baseline to Wk128: 0.79; % of pts with radiographic non-progression at Wk24: 84.6%, Wk128: 73.2%). Clinical improvements were maintained in the dose reduction population (400mg to 200mg Q2W +MTX; N=288) from the first CZP 200mg treatment (DAS28[ESR]=3.5) through 132 wks of CZP 200mg Q2W (DAS28[ESR]=3.6).
Conclusion:
In pts with active RA despite MTX, CZP+MTX maintained reduction in signs and symptoms of RA with a favorable long-term risk-benefit ratio.
References:
1. Smolen J.S. Ann Rheum Dis 2009;68:797-804; 2. Smolen J.S. Arthritis Rheum 2010;62(Suppl10):1806.
Disclosure:
J. S. Smolen,
UCB Pharma,
2,
UCB Pharma,
5;
R. van Vollenhoven,
Abbvie, BMS, GSK, MSD, Pfizer, Roche, UCB Pharma,
2,
Abbott, BMS, GSK, Lilly, MSD, Pfizer, Roche, UCB Pharma,
5;
A. Kavanaugh,
Abbott, Amgen, BMS, Janssen, Pfizer, Roche, UCB Pharma,
2;
V. Strand,
UCB Pharma,
5;
J. Vencovsky,
Abbott, MSD, Pfizer, Roche, UCB Pharma,
5,
MSD, Pfizer, Roche, UCB Pharma,
8;
M. H. Schiff,
UCB Pharma,
5,
UCB Pharma,
2;
R. Landewé,
Abbott, Ablynx, Amgen, Astra-Zeneca, Bristol-Myers Squibb, Centocor, GlaxoSmithKline, Novartis, Merck, Pfizer, Roche, Schering-Plough, UCB Pharma, Wyeth,
5,
Abbott, Amgen, Centocor, Novartis, Pfizer, Roche, Schering-Plough, UCB Pharma, Wyeth ,
2,
Abbott, Amgen, Bristol-Myers Squibb, Centocor, Merck, Pfizer, Roche, Schering-Plough, UCB Pharma, Wyeth,
8;
B. Haraoui,
Abbott, Amgen, BMS, Janssen, Pfizer, Roche and UCB Pharma,
2,
Abbott, Amgen, BMS, Janssen, Pfizer, Roche and UCB Pharma,
8,
Abbott, Amgen, BMS, Janssen, Pfizer, Roche and UCB Pharma,
5;
S. Walker,
UCB Pharma,
3;
D. van der Heijde,
AbbVie, Amgen, AstraZeneca, Augurex, BMS, Celgene, Centocor, Chugai, Covagen, Daiichi, Eli-Lilly, GSK, Janssen Biologics, Merck, Novartis, Novo-Nordisk, Otsuka, Pfizer, Roche, Sanofi-Aventis, Schering-Plough, UCB Pharma, Vertex ,
5,
Director of Imaging Rheumatology bv.,
4.
« Back to 2013 ACR/ARHP Annual Meeting
ACR Meeting Abstracts - https://acrabstracts.org/abstract/long-term-safety-and-efficacy-of-certolizumab-pegol-in-combination-with-methotrexate-in-the-treatment-of-rheumatoid-arthritis-5-year-results-from-a-24-week-randomized-controlled-trial-and-open-label/