Session Information
Session Type: Poster Session C
Session Time: 1:00PM-3:00PM
Background/Purpose: Rheumatic diseases substantially affect the lives of patients, with complex associations between disease severity and self-perceived health status. In this regard, the Coping with Rheumatic Stressors (CORS) questionnaire was developed to measure how patients with rheumatoid arthritis cope with stressors such as pain or dependence. There is no validated instrument to measure coping in axial spondyloarthritis (axSpA) and therefore the adaptation of the CORS would be of great value. The purpose of this study was to cross-culturally adapt the CORS into Spanish and to test the conceptual equivalence of the translated version in patients with axSpA.
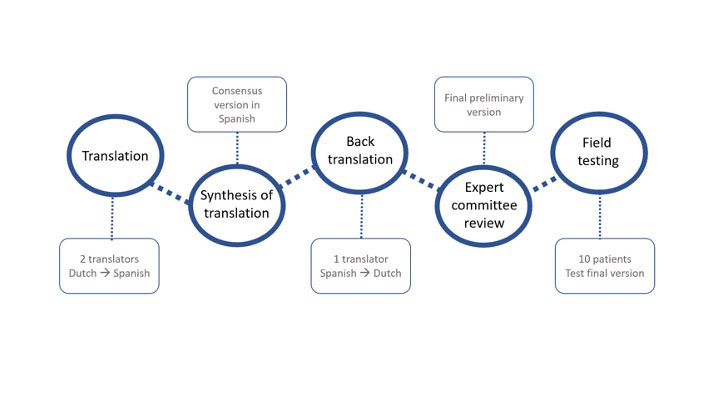
Methods: A translation of the CORS into Spanish was performed, followed by a back-translation into Dutch, following forward-backward procedure as described by Beaton1(Figure). Two bi-lingual translators (native speakers for Spanish) produced independent forward translations of the item content, response options, and instructions of the CORS into Spanish. Both versions were harmonized in a consensual version. Another translator (native speaker for Dutch), not informed of the concepts used in the questionnaire, back translated the synthesized version into Dutch. An expert committee including all translators, one methodologist and a rheumatologist, held a meeting and reached consensus on discrepancies to develop a pre-final version of the Spanish CORS. The field test with cognitive debriefing involved a sample of 10 patients with axSpA covering the full spectrum of the disease and with different sociodemographic backgrounds.
Results: The translation process of the CORS was completed following the forward-backward procedure, after discussion of the discrepancies throughout the process. The first translation was done without major complications. However, several discrepancies appeared in the back-translation, in which there were minor modifications in the wording in one response option (“muchas veces” to “muy a menudo”) and 15 questionnaire items. As an example, “Ik ga de deur uit”, literally meaning “I go out by the door”, was initially translated as such (“salgo por la puerta”); however, it conceptually represents “I go away”, and it was adapted like this (“me voy a la calle”). Thus, a pre-final consensus version of the CORS was agreed by the expert committee. This pre-final version was field tested in 10 patients with axSpA: mean age (SD) was 38.9 (14.4) years, 7 patients were male, 6 had radiographic axSpA, and 9 were HLA-B27+. The Spanish questionnaire appeared clear and understandable to all patients. However, some minor modifications were proposed in some items (Table). As a result of the cognitive debriefing, two changes were implemented (one instruction and one item), whereas two other suggestions did not lead to any change due to minor wording discrepancies with similar conceptual equivalence. The final version of the Spanish CORS is shown at https://www.dropbox.com/s/4j0q1y06krl2ek2/CORS_FINAL_translation_Spanish.docx.
Conclusion: The Spanish version of the CORS showed good cross-cultural validity and good face validity in patients with axSpA according to the field test. Before the Spanish CORS is implemented, further validation is in progress to test the psychometric properties of the instrument.
To cite this abstract in AMA style:
Navarro-Compán V, Benavent D, Jochems A, Pascual-Salcedo D, Jochems G, Ramiro S, van Lankveld W, Plasencia-Rodriguez C. Translation and Cross-cultural Adaptation of the CORS into Spanish [abstract]. Arthritis Rheumatol. 2022; 74 (suppl 9). https://acrabstracts.org/abstract/translation-and-cross-cultural-adaptation-of-the-cors-into-spanish/. Accessed .« Back to ACR Convergence 2022
ACR Meeting Abstracts - https://acrabstracts.org/abstract/translation-and-cross-cultural-adaptation-of-the-cors-into-spanish/