Session Information
Session Type: Abstract Submissions (ACR)
Background/Purpose: This analysis evaluated the long-term safety of rituximab (RTX) in rheumatoid arthritis (RA) patients in a global clinical trial program.
Methods: Pooled observed case analysis of safety data from patients with moderate to severe active RA treated with RTX+MTX. Patients were re-treated based on physician’s determination of clinical need and evidence of active disease (defined as either SJC and TJC ≥8 or DAS28 ≥2.6). Subgroup analysis of patients with follow-up >5 years was undertaken. Pooled data from patients who received placebo during placebo-controlled study periods were also analyzed.
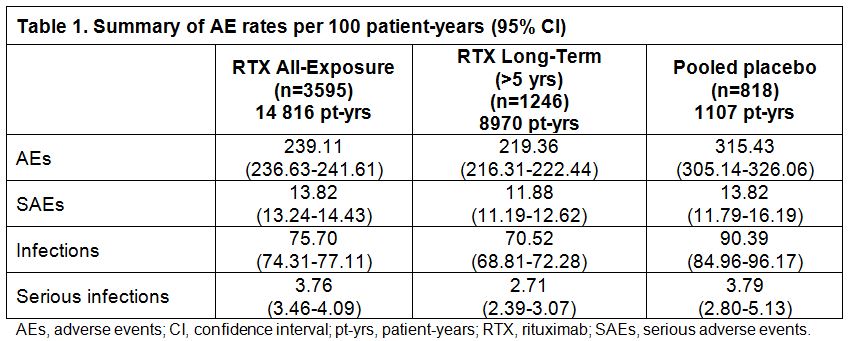
Results : As of Sep 2012, 3595 patients (All-Exposure population) had received up to 20 courses of RTX over the 11-year observation period (14 816 pt-yrs). Of these patients, 1246 had >5 years follow-up. The placebo population comprised 818 patients (1107 pt-yrs) with a mean follow-up of 1–1.5 years. In the All-Exposure population, infusion-related reaction (IRR) was the most frequent adverse event (AE); most were grade 1 or 2, were rarely serious (0.5%), and primarily occurred following the 1st infusion of the 1st course (799/3595 patients; 22%). Rates per 100 pt-yrs for AEs, serious AEs (SAEs), and infections were not increased when compared to placebo (Table). Overall serious infection rates in RTX-treated patients were comparable to those observed in the placebo population. Pneumonia was the most frequently reported serious infection (2% of RTX patients). There were no cases of hepatitis B reactivation. Serious opportunistic infections were rare (0.05/100 pt-yrs in RTX patients vs 0.09/100 pt-yrs in placebo). One confirmed case of PML in the RA clinical trial program has been reported, as previously described.1 Following RTX treatment, low immunoglobulin (Ig) concentrations (particularly IgM, less often IgG) were observed. For both Ig classes, serious infection rates were similar before and during/after development of low Ig. No increased risk of malignancy over time or course was evident, and MI rates (0.39/100 pt-yrs) were consistent with rates in the general RA population (0.48–0.59/100 pt-yrs).2
Conclusion: These long-term data from 3595 patients treated with RTX over 11 years (14 816 pt-yrs) of follow-up in clinical trials confirm that RTX remains well tolerated over time and multiple courses, with a consistent safety profile. No new safety signals were observed with increasing duration of exposure. Apart from IRRs and low Ig (where there was a lack of placebo comparator), the overall safety profile of RTX remains similar to that for the pooled placebo population and is consistent with published data for moderate to severe RA and with previous analyses of this patient cohort.3
1. Fleischmann RM. Arthritis Rheum. 2009;60:3225.
2. British Society for Rheumatology Biologics Register, 2007.
3. van Vollenhoven RF, et al. Ann Rheum Dis. 2012 Nov 7. [Epub ahead of print].
Disclosure:
R. van Vollenhoven,
AbbVie, BMS; GSK, Merck, Pfizer, Roche, UCB,
2,
AbbVie, BMS, GSK, Lilly, Merck, Pfizer, Roche, UCB, Vertex,
5;
P. Emery,
Pfizer, Merck, Abbott, BMS, Roche, Novartis, UCB,
5;
C. O. Bingham III,
Roche, Genentech, Biogen Idec,
2,
Roche, Genentech,
5;
E. Keystone,
Abbott, Amgen, AstraZeneca, Baylis, Bristol-Myers Squibb, F. Hoffman La-Roche, Janssen, Lilly, Novartis, Pfizer, Sanofi-Aventis, UCB,
2,
Abbott, AstraZeneca, Biotest, Bristol-Myers Squibb, F. Hoffman La-Roche, Genentech, Janssen, Lilly, Merck, Nycomed, Pfizer, UCB,
5,
Abbott, Amgen, AstraZeneca, Bristol-Myers Squibb Canada, F. Hoffman La-Roche, Janssen, Pfizer, UCB,
8;
R. Fleischmann,
Genentech, Roche, Abbott, Amgen, UCB, Pfizer, BMS, Lilly, Sanofi-Aventis, Lexicon, MSD, Novartis, Biogen Idec, Astellas, AstraZeneca, Janssen,
2,
Roche, Abbott, Amgen, UCB, Pfizer, BMS, Lilly, Sanofi-Aventis, Lexicon, Novartis, Astellas, AstraZeneca, Janssen, HGS,
5;
D. Furst,
Abbott, Actelion, Amgen, BMS, Gilead, GSK, NIH, Novartis, Pfizer, Roche/Genentech, UCB,
2,
Abbott, Actelion, BMS, Biogen Idec, Centocor, CORRONA, Gilead, GSK, NIH, Novartis, Pfizer, Roche/Genentech, UCB,
5,
Abbott, Actelion, UCB,
8;
E. W. Hessey,
Roche Products Ltd,
3;
A. Mehbob,
Roche Products Ltd.,
3;
P. B. Lehane,
Roche Products Ltd.,
3.
« Back to 2013 ACR/ARHP Annual Meeting
ACR Meeting Abstracts - https://acrabstracts.org/abstract/long-term-safety-of-rituximab-pooled-analysis-of-the-rheumatoid-arthritis-global-clinical-trial-program-over-11-years/