Session Information
Date: Sunday, November 13, 2022
Title: Vasculitis – ANCA-Associated Poster II: Treatment Efficacy, Clinical Outcomes, Biomarkers
Session Type: Poster Session B
Session Time: 9:00AM-10:30AM
Background/Purpose: Patients with eosinophilic granulomatosis with polyangiitis (EGPA) can have vasculitic or eosinophilic phenotypes. The MIRRA study demonstrated that patients with EGPA spent more time in remission and had reduced oral corticosteroid (OCS) use with mepolizumab vs placebo (PBO).This analysis evaluated mepolizumab in patients with a vasculitic phenotype enrolled in MIRRA.
Methods: MIRRA was a Phase III, multicentre, double-blind, parallel-group trial in adults with relapsing/refractory EGPA and ≥4 weeks stable OCS treatment. Patients were randomised to standard of care plus mepolizumab (300 mg SC Q4W) or PBO for 52 weeks. Primary endpoints: accrued weeks of remission (defined as Birmingham Vasculitis Activity Score [BVAS] of 0 and OCS dose ≤4 mg/day prednisolone or equivalent) over 52 weeks categorised in weeks (0, >0 to < 12, 12 to < 24, 24 to < 36. ≥36 weeks), and proportion in remission at both Weeks 36 and 48. This post hoc analysis evaluated these endpoints according to antineutrophil cytoplasmic antibody (ANCA) history (current or previous positive test for myeloperoxidase[MPO]/proteinase 3[PR3]-ANCA at study baseline vs no history of a positive MPO/PR3-ANCA test at baseline), baseline BVAS (=0 vs >0) and Vasculitis Damage Index (VDI) score (< 5 vs ≥5). Types of disease relapse (vasculitis [BVAS >0], asthma [active asthma symptoms and/or signs with worsening in Asthma Control Questionnaire-6 score] and sinonasal [active nasal and/or sinus disease with worsening in ≥1 sinonasal symptom questions]) reported during the treatment period were described. EGPA disease characteristics focusing on vasculitic components were assessed.
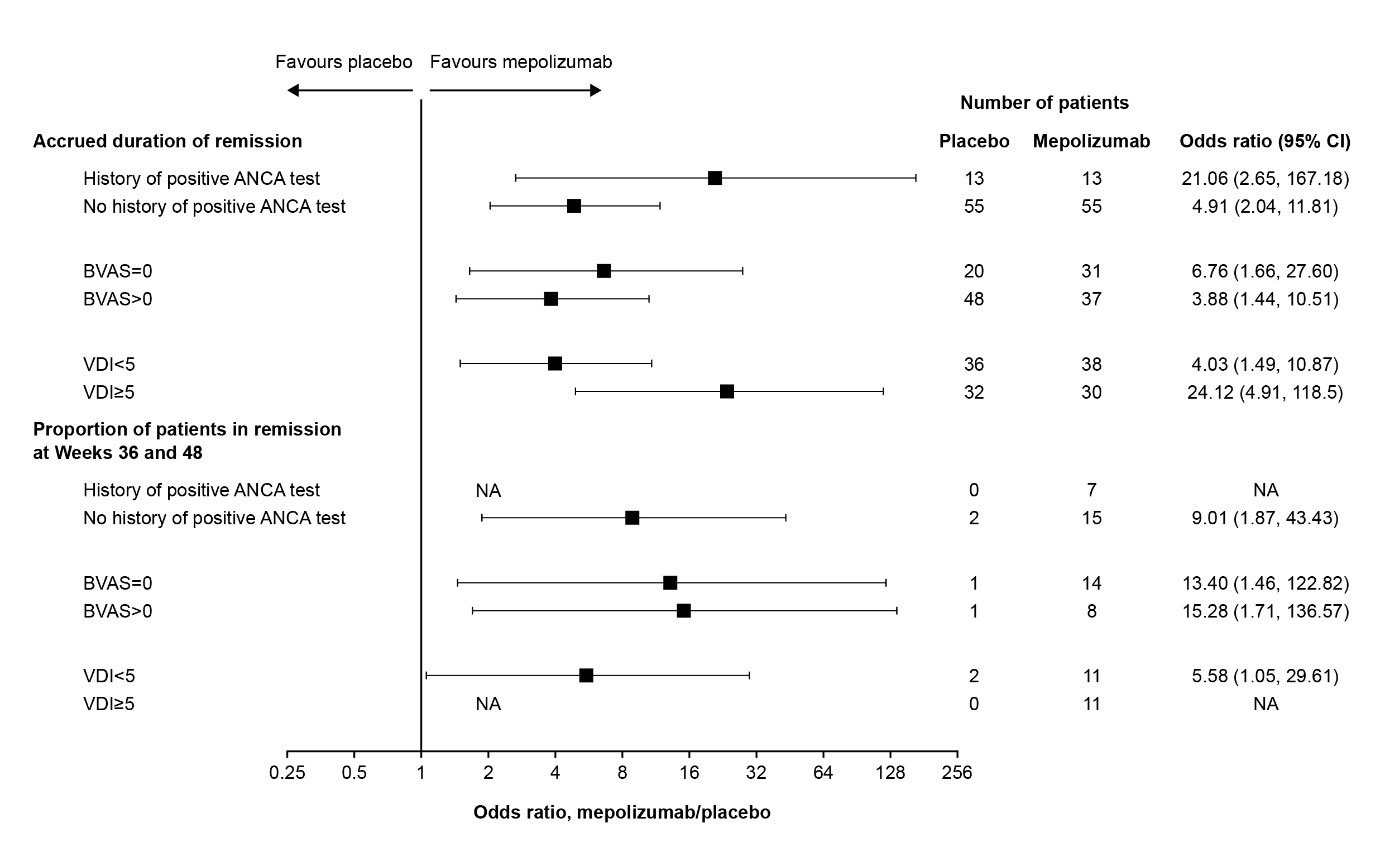
Results: Of 136 patients, 26 (19%) had a history of a positive ANCA test at study baseline; 110 (81%) did not. In addition, 51 (38%) had BVAS =0 at baseline while 85 (63%) had BVAS >0; 74 (54%) had VDI < 5 at baseline and 62 (46%) had VDI ≥5. Accrued remission duration was greater with mepolizumab vs PBO, irrespective of ANCA positivity, baseline BVAS or baseline VDI (Figure). Across all subgroups, a larger proportion achieved remission at both Weeks 36 and 48 with mepolizumab vs PBO (Figure). Among those receiving mepolizumab, the numbers (proportion) achieving remission at both Weeks 36 and 48 were: 7 (54%) for those with a history of an ANCA-positive test and 15 (27%) for those without a history; 14 (45%) in the BVAS =0 and 8 (22%) in the BVAS >0 groups; 11 (29%) in the VDI < 5 and 11 (37%) in the VDI ≥5 groups. Mepolizumab reduced all types of disease relapse assessed during the treatment period, including vasculitis, asthma and sinonasal relapses, vs PBO. Vasculitic characteristics including neuropathy, glomerulonephritis, alveolar haemorrhage, palpable purpura and ANCA positivity were generally similar among those who did and did not achieve remission during the study.
Conclusion: Mepolizumab is associated with clinical benefits in EGPA, including in patients with and without a vasculitic phenotype.
Funding: GSK (MEA115921; NCT02020889); the Division of Intramural Research, NIAID, NIH funded in part time spent on this abstract by PK.
Abstract previously presented at EULAR 2022 and adapted with permission from BMJ.
ANCA, antineutrophil cytoplasmic antibody; BVAS, Birmingham Vasculitis Activity Score; CI, confidence interval; NA, data not available – estimate could not be calculated owing to a lack of patients in the placebo group achieving remission at Weeks 36 and 48; VDI, Vasculitis Damaging Index.
To cite this abstract in AMA style:
Terrier B, Jayne D, Hellmich B, Bentley J, Steinfeld J, Yancey S, Kwon N, Akuthota P, Khoury P, Baylis L, Wechsler M. Efficacy of Mepolizumab in Patients with Eosinophilic Granulomatosis with Polyangiitis and a Vasculitic Phenotype [abstract]. Arthritis Rheumatol. 2022; 74 (suppl 9). https://acrabstracts.org/abstract/efficacy-of-mepolizumab-in-patients-with-eosinophilic-granulomatosis-with-polyangiitis-and-a-vasculitic-phenotype/. Accessed .« Back to ACR Convergence 2022
ACR Meeting Abstracts - https://acrabstracts.org/abstract/efficacy-of-mepolizumab-in-patients-with-eosinophilic-granulomatosis-with-polyangiitis-and-a-vasculitic-phenotype/