Session Information
Date: Sunday, November 13, 2022
Title: RA – Treatment Poster II
Session Type: Poster Session B
Session Time: 9:00AM-10:30AM
Background/Purpose: Methotrexate (MTX) significantly decreases the vaccine response to pneumococcal and seasonal influenza vaccines and temporarily discontinuing MTX for 2 weeks in patients with rheumatoid arthritis (RA) on a stable dose of MTX significantly increased immunogenicity. This study aimed to determine whether discontinuing methotrexate (MTX) for 1 week after seasonal influenza vaccination is non-inferior to discontinuing for 2 weeks in RA patients with regards to immediate and long-term vaccine response.
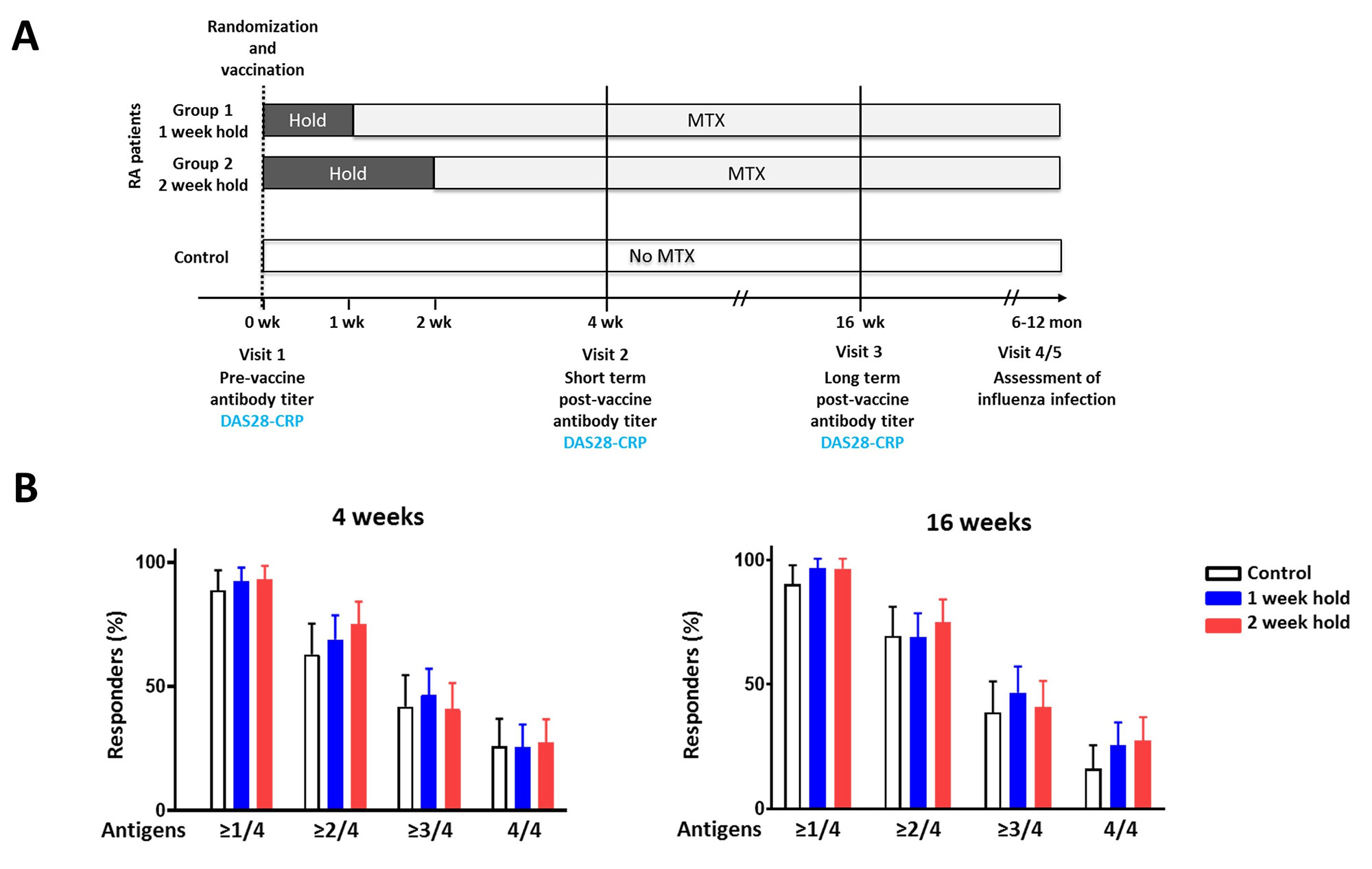
Methods: In this prospective randomized parallel-group multi-center non-inferiority trial study, RA patients on a stable dose of MTX were randomly assigned at a ratio of 1:1 to hold MTX for 1 week or 2 weeks after receiving the quadrivalent 2021–2022 seasonal influenza vaccine containing H1N1, H3N2, B-Yamagata, and B-Victoria strains. The primary outcome was the proportion of patients with a satisfactory vaccine response, which was defined as ≥4-fold increase in hemagglutination inhibition antibody titers against two or more of the four vaccine strains 4 weeks after vaccination. Secondary outcomes include positive response and antibody titers at 4 and 16 weeks after vaccination. Controls without autoimmune disease were included as a reference.
Results: The modified intention-to-treat population included 90 patients in the 1 week MTX hold group and 88 patients in the 2 week MTX hold group. The proportion of satisfactory vaccine responses did not differ between the groups at 4 weeks (68.9% vs. 75.0%, p = 0.364) and at 16 weeks (69.6% vs. 70.3%, p=0.915). The proportion of patients reaching seroprotection and the rise in antibody titer were similar between the groups at 4 and 16 weeks. Vaccine responses in RA patients and controls were similar.
Conclusion: Temporarily discontinuing MTX for 1 week is non-inferior to MTX discontinuation for 2 weeks after vaccination to induce an immediate and long-term satisfactory vaccine response to a seasonal influenza vaccine in patients with RA on a stable dose of MTX.
(A) Study design. (B) The percentage of patients achieving a satisfactory vaccine response, defined as ≥4-fold increase in hemagglutination inhibition (HI) antibody titer 4 weeks and 16 weeks after vaccination against one or more, two or more , three or more, and four vaccine strains. The error bars represent the 95% confidence interval. The 1 week and 2 week MTX hold groups were compared by a chi-square test. The groups did not differ in regard to vaccine response (p >0.05).
To cite this abstract in AMA style:
Park J, Lee Y, Shin K, Kang E, Ha Y, Kim M, Park J, CHOI S, Kim M, Jung J, Kim J, Winthrop K, Lee E. Immediate and Long-term Effects of the MTX Discontinuation for 1 vs. 2 Weeks on Vaccine Response to Seasonal Influenza Vaccine: A Non-inferiority Randomized Controlled Trial [abstract]. Arthritis Rheumatol. 2022; 74 (suppl 9). https://acrabstracts.org/abstract/immediate-and-long-term-effects-of-the-mtx-discontinuation-for-1-vs-2-weeks-on-vaccine-response-to-seasonal-influenza-vaccine-a-non-inferiority-randomized-controlled-trial/. Accessed .« Back to ACR Convergence 2022
ACR Meeting Abstracts - https://acrabstracts.org/abstract/immediate-and-long-term-effects-of-the-mtx-discontinuation-for-1-vs-2-weeks-on-vaccine-response-to-seasonal-influenza-vaccine-a-non-inferiority-randomized-controlled-trial/