Session Information
Session Type: Poster Session B
Session Time: 9:00AM-10:30AM
Background/Purpose: Randomized controlled trials (RCTs) provide high-quality evidence for treatment efficacy, but many RCTs remain unpublished. The objective of this study was to describe the proportion of unpublished RCTs in rheumatology and to identify factors associated with publication.
Methods: Registered RCTs for 5 rheumatic diseases (systemic lupus erythematosus, vasculitis, spondyloarthritis, Sjögren’s syndrome, and psoriatic arthritis) with over 30 months since study completion were identified using ClinicalTrials.gov. Index publications were identified by NCT ID numbers and structured text searches of publication databases. The results of unpublished studies were identified in abstracts and press releases; reasons for non-publication were assessed by surveying corresponding authors.
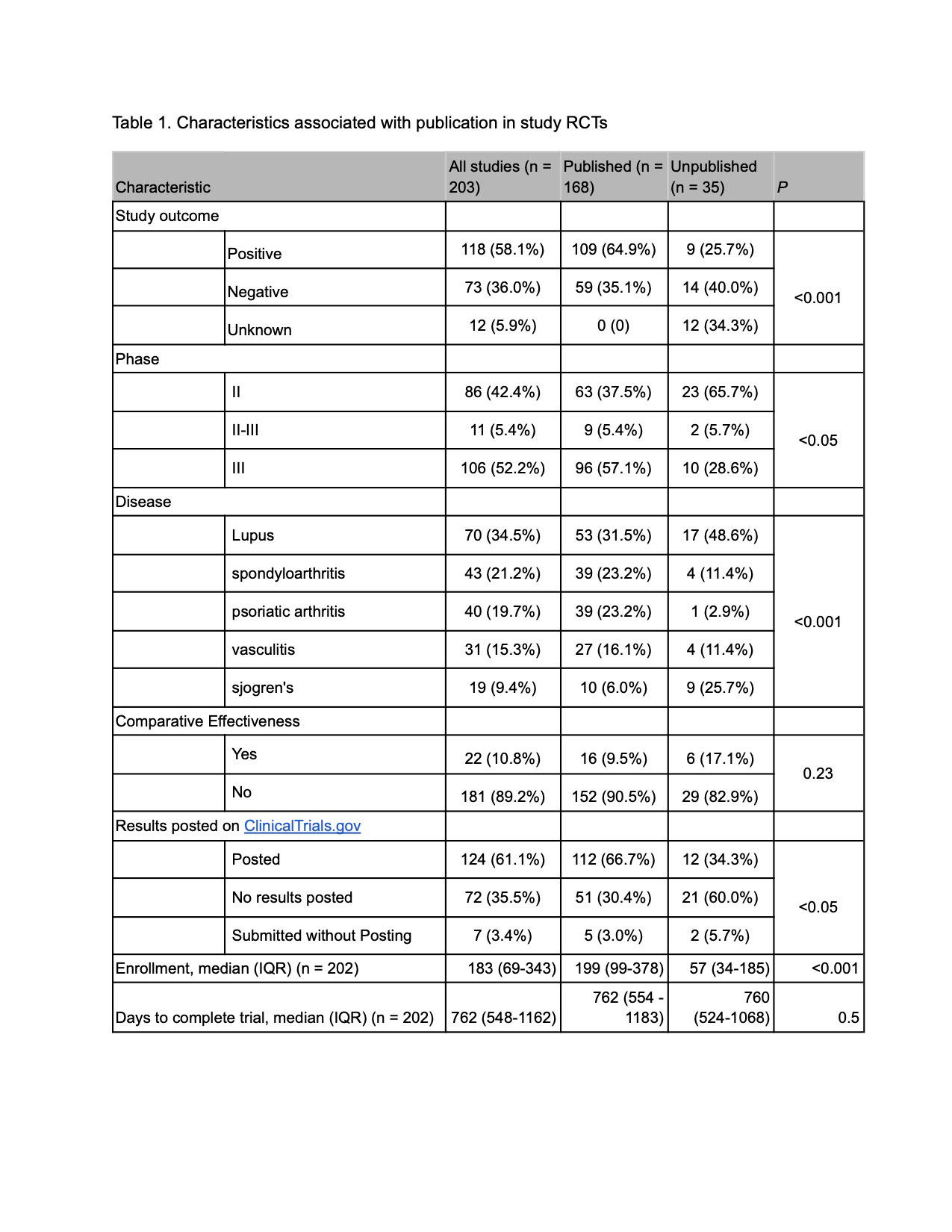
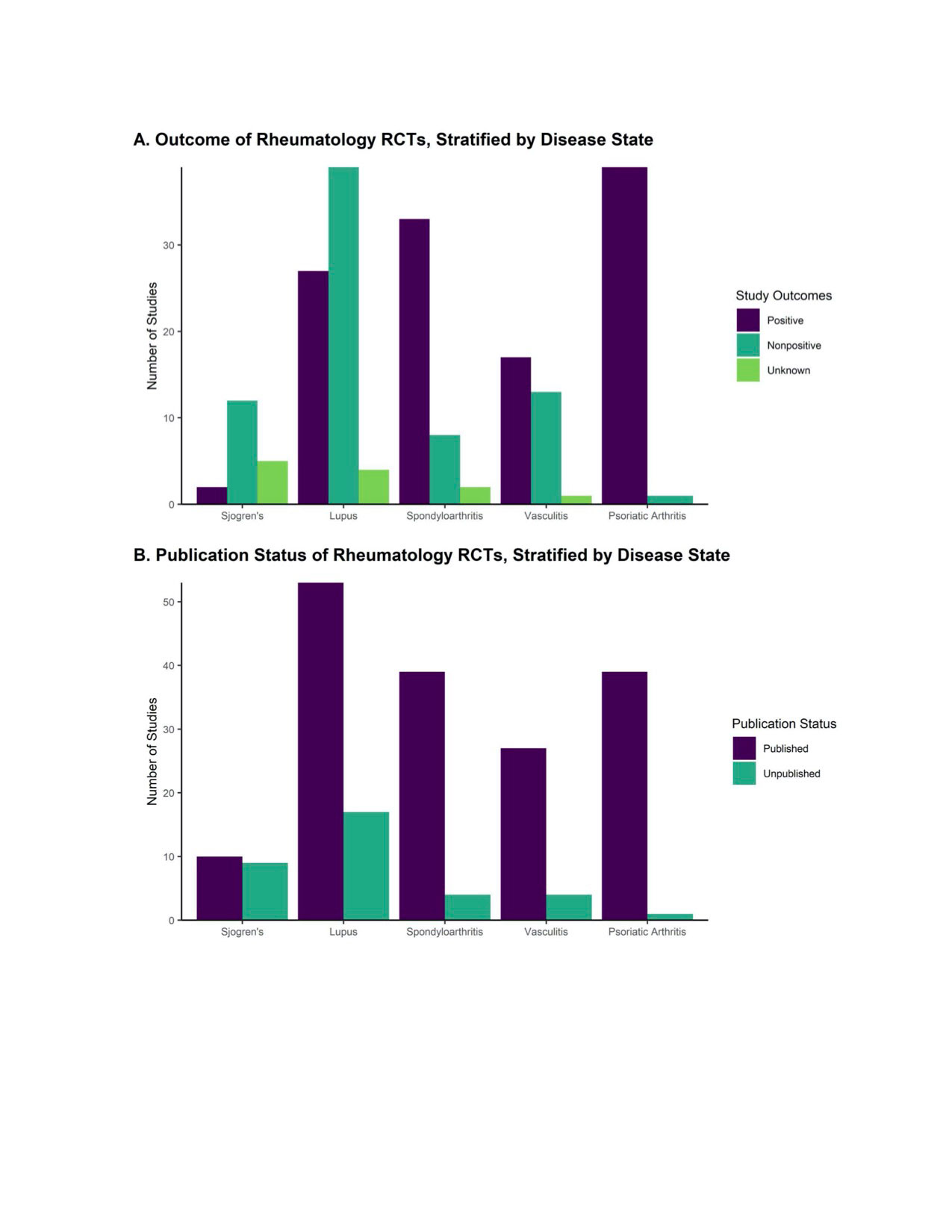
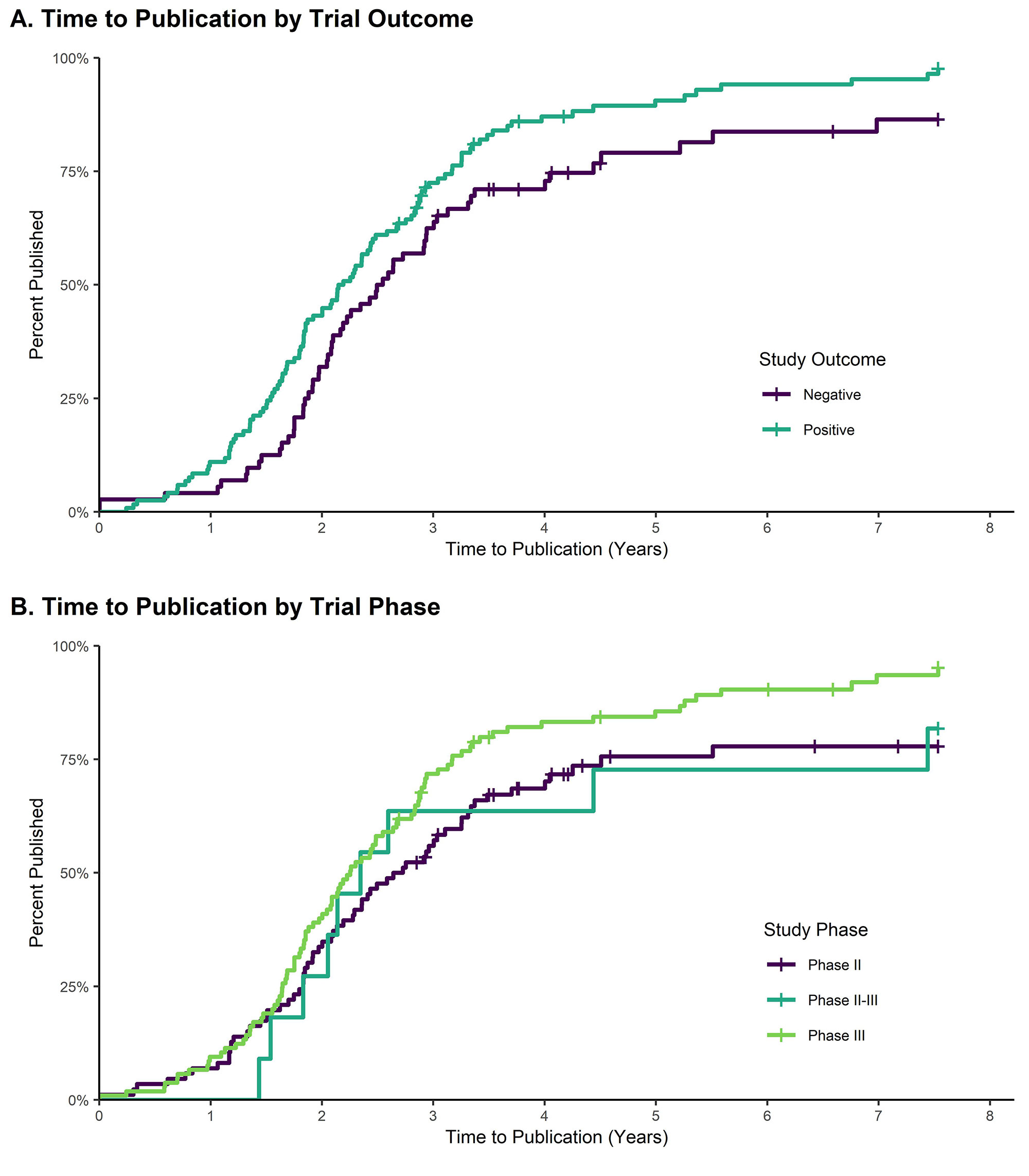
Results: Out of 203 studies that met eligibility criteria, 17.2% remained unpublished, representing data from 4,281 trial participants. Higher proportions of published trials were phase 3 RCTs (57.1% vs 28.6% unpublished, p < 0.05) or had a positive primary outcome measure (64.9% vs 25.7% unpublished, p < 0.001), and in a multivariable cox proportional hazards model, a positive outcome was independently associated with publication (HR 1.55, CI 1.09-2.22). Corresponding authors of unpublished trials commonly cited ongoing preparation of the manuscript (50.0%), sponsor/funder issues (40.0%), and unimportant/negative result (20.0%) as reasons for lack of publication.
Conclusion: Nearly one in five RCTs in rheumatology remain unpublished two years after trial completion, and publication is associated with positive primary outcome measures. Efforts to encourage universal publication of rheumatology RCTs and reanalysis of previously unpublished trials should be undertaken.
To cite this abstract in AMA style:
Pedersen C, Putman M, Valley E, Henry K, Duarte-Garcia A, Singla S, Tai S. Unpublished Clinical Trials of Major Rheumatic Diseases [abstract]. Arthritis Rheumatol. 2022; 74 (suppl 9). https://acrabstracts.org/abstract/unpublished-clinical-trials-of-major-rheumatic-diseases/. Accessed .« Back to ACR Convergence 2022
ACR Meeting Abstracts - https://acrabstracts.org/abstract/unpublished-clinical-trials-of-major-rheumatic-diseases/