Session Information
Session Type: Poster Session A
Session Time: 1:00PM-3:00PM
Background/Purpose: Glucocorticoids (GC) are the first line treatment for myositis patients. They improve muscle strength, yet muscle recovery is generally partial and long lasting treatment leads to steroid myopathy. Thus, myositis care has to be improved. GC effects are mediated by the glucocorticoid receptor (GR), which is expressed in both immune cells and myofibres, but the cells mediating therapeutic response in myositis remain to be determined. Our team has recently shown that muscle fibres immuno-metabolic modifications participate to muscle weakness and perpetuation of the disease (Meyer et al. 2017).
The aim of this study was to unravel the role of skeletal muscle fibres in the therapeutic effect of GC in myositis.
Methods: To study cell-specific effect of GC, we generated transgenic mice carrying two LoxP sites within the GR gene and expressing the tamoxifen-dependent Cre-ERT2 recombinase selectively in skeletal muscle fibre (HSA-CreERT2/GR L2/L2). Experimental myositis (EM) was induced in eight to ten week-old C57BL/6J mice by skeletal muscle fast-type C protein injection as previously described (Sugihara et al 2007).
Tamoxifen was administered 9 days (D) after immunization for 5 D to induce GR ablation selectively in myofibre (GR(i)skm-/- mice). Then, prednisone (PDN) was administered 14 D after immunization at 1 mg/kg/day for 7 D. Mice were euthanized 21 D after EM induction.
Similar treatments were applied to GR L2/L2 mice not expressing Cre-ERT2 recombinase (controls). EM-GR L2/L2 treated by PDN or vehicle and EM-GR(i)skm-/- treated by PDN or vehicle were compared.
Grip test was performed at D0, before the 1st PDN administration (D14) and the day before sacrifice (D20). Creatine-kinase (CK) activity assay in serum, muscle histology, immune-cell phenotyping using flow cytometry and gastrocnemius transcriptomic analysis were run. Transcriptomic data were validated in independent mice cohorts, in vitro and on human muscle biopsies.
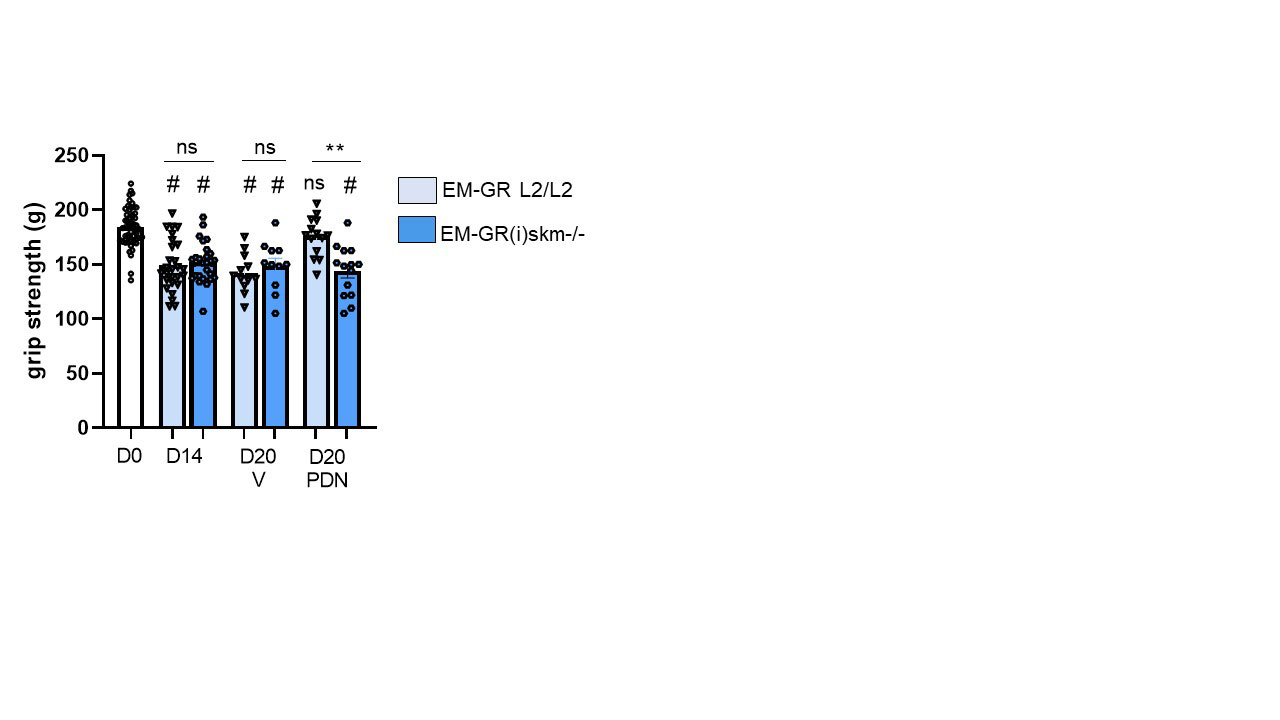
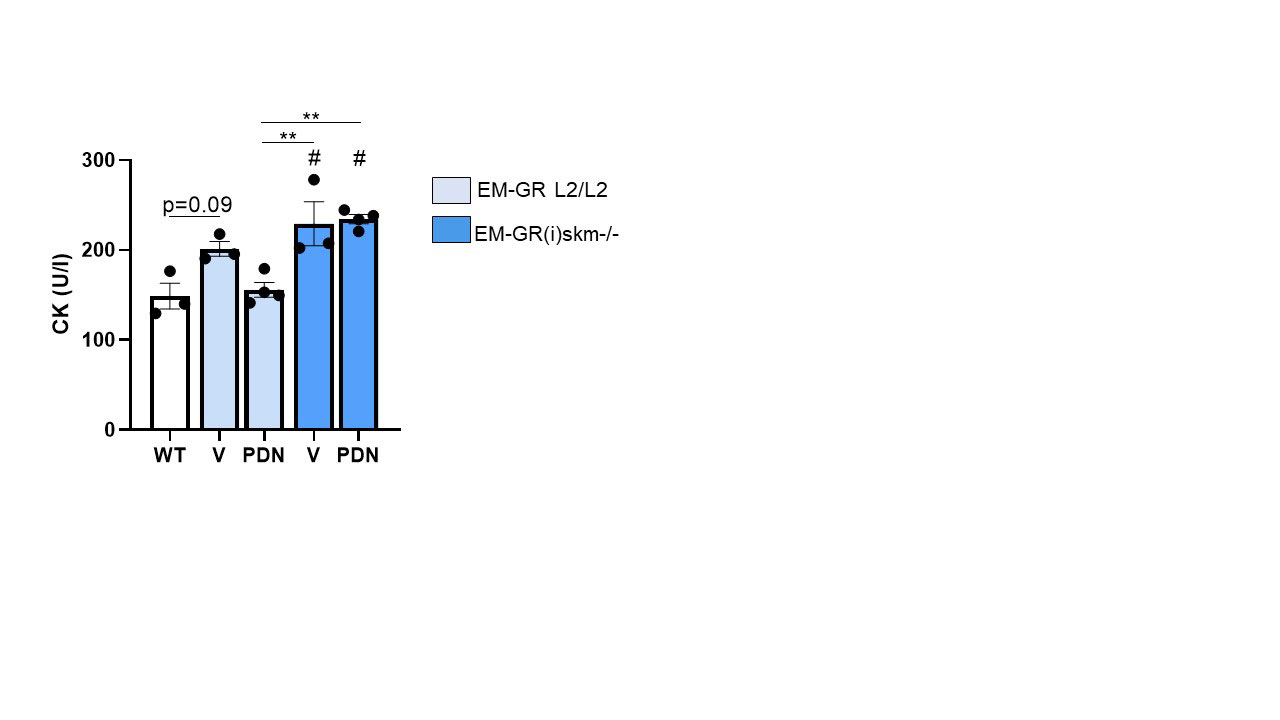
Results: Muscle strength was decreased by 20% from D14 to D20 in vehicle-treated EM-GR L2/L2 and EM-GR(i)skm-/- mice. EM-GR L2/L2 mice recovered muscle strength after PDN treatment. Conversely, PDN did not improve muscle strength in EM-GR(i)skm-/- mice (figure 1). Consistently, at the end of experiment, CK serum levels were increased in both vehicle-treated EM-GR L2/L2 and EM-GR(i)skm-/- mice. In EM-GR L2/L2 mice, PDN restored CK normal values. This therapeutic effect was abolished in EM-GR(i)skm-/- mice treated by PDN (figure 2).
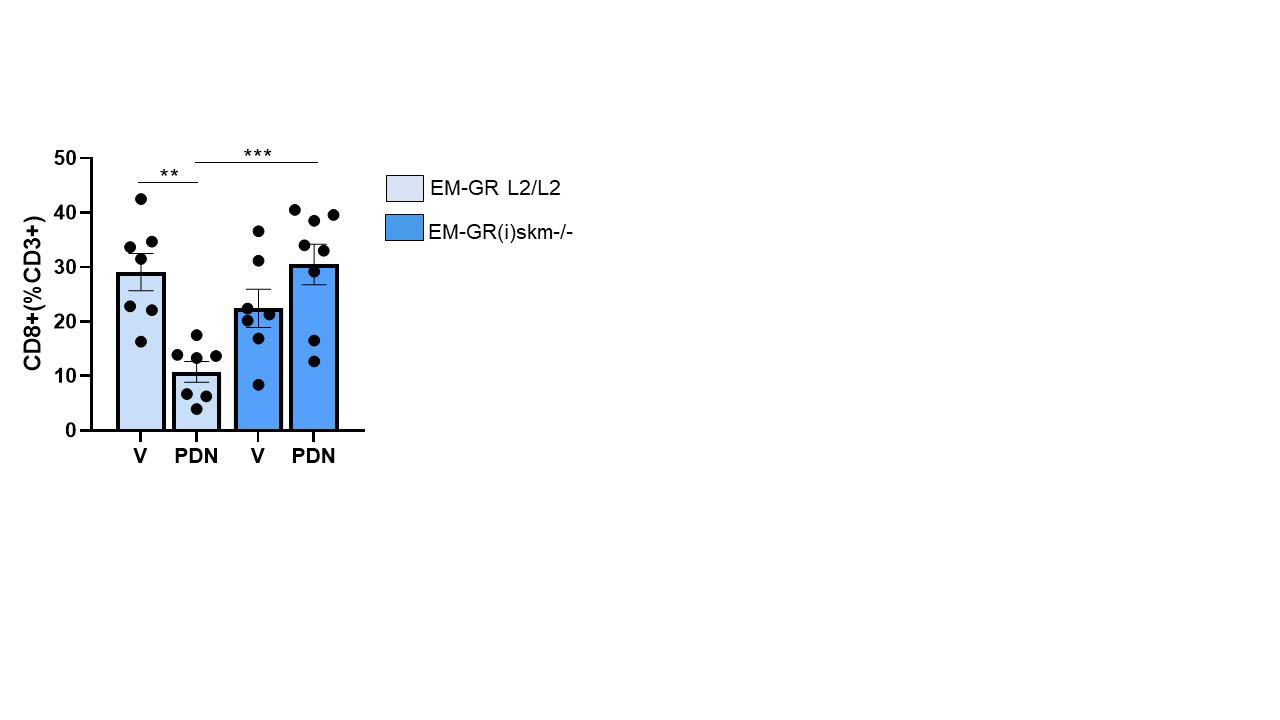
Muscle flow cytometry analysis showed a 3-fold decrease in CD8+ T-cells in the EM-GRL2/L2 mice treated by PDN compared to vehicle group (p=0,003). This PDN effect was suppressed in EM-GR(i)skm-/- mice (figure 3).
At transcriptomic level, the top 10 deregulated genes of the fibre-dependent PDN signature belonged to muscle fibre metabolism and T cells recruitment pathways. Interestingly, some mediators produced by myofibre have been identified as candidates for a paracrine immunomodulatory effect.
Conclusion: Skeletal muscle fibres play a critical role in the GC therapeutic effect in myositis through a polarization of inflammatory infiltrate toward an anti-inflammatory response by a paracrine mechanism.
To cite this abstract in AMA style:
Giannini M, Rovito D, Debrut L, Keime C, Lannes B, Charles A, Duteil D, Geny B, Metzger D, Laverny G, Meyer A. Muscle Fibres Play a Critical Role in Therapeutic Response of Myositis to Glucocorticoids Through Polarisation of the Inflammatory Infiltrate by a Paracrine Mechanism [abstract]. Arthritis Rheumatol. 2022; 74 (suppl 9). https://acrabstracts.org/abstract/muscle-fibres-play-a-critical-role-in-therapeutic-response-of-myositis-to-glucocorticoids-through-polarisation-of-the-inflammatory-infiltrate-by-a-paracrine-mechanism/. Accessed .« Back to ACR Convergence 2022
ACR Meeting Abstracts - https://acrabstracts.org/abstract/muscle-fibres-play-a-critical-role-in-therapeutic-response-of-myositis-to-glucocorticoids-through-polarisation-of-the-inflammatory-infiltrate-by-a-paracrine-mechanism/