Session Information
Session Type: Poster Session A
Session Time: 1:00PM-3:00PM
Background/Purpose: IgG4-related disease (IgG4-RD) is a rare, heterogenous and potentially severe disease. An open-label trial demonstrated efficacy of rituximab (RTX) in IgG4-RD. Recently, four distinct phenotypes of IgG4-RD were identified. There is a paucity of studies investigating RTX efficacy across these phenotypes. We aimed to investigate efficacy and safety of RTX in IgG4-RD and segregated by phenotypes.
Methods: This is an open-label non-randomized single center observational study. All patients with IgG4-RD (diagnosed by expert opinion) at the Oslo University Hospital treated with ≥ 1 dose of RTX with 12 months follow-up were included. Two experts (JV, ØMi) assigned patients to phenotypes. Glucocorticoid (GC) treatment was allowed. We measured disease activity by the IgG4- RD Responder Index (IgG4-RD RI) at baseline, 6 months, and 12 months. We defined a composite primary outcome consisting of two measures; (i) reduced disease activity (i.e., ≥2 points improvement in IgG4-RD RI from baseline and/or IgG4-RD RI score 0 at follow-up), and (ii) no disease flares (i.e., no ≥2 points worsening of IgG4-RD RI and no need to increase GC dose) at 6 months. Secondary outcomes were (a) reduced disease activity at months 6 or 12, (b) remission (IgG4-RD RI score 0 and GC dose ≤ 7.5 mg) at 6 or 12 months and (c) safety. Descriptive statistics were applied.
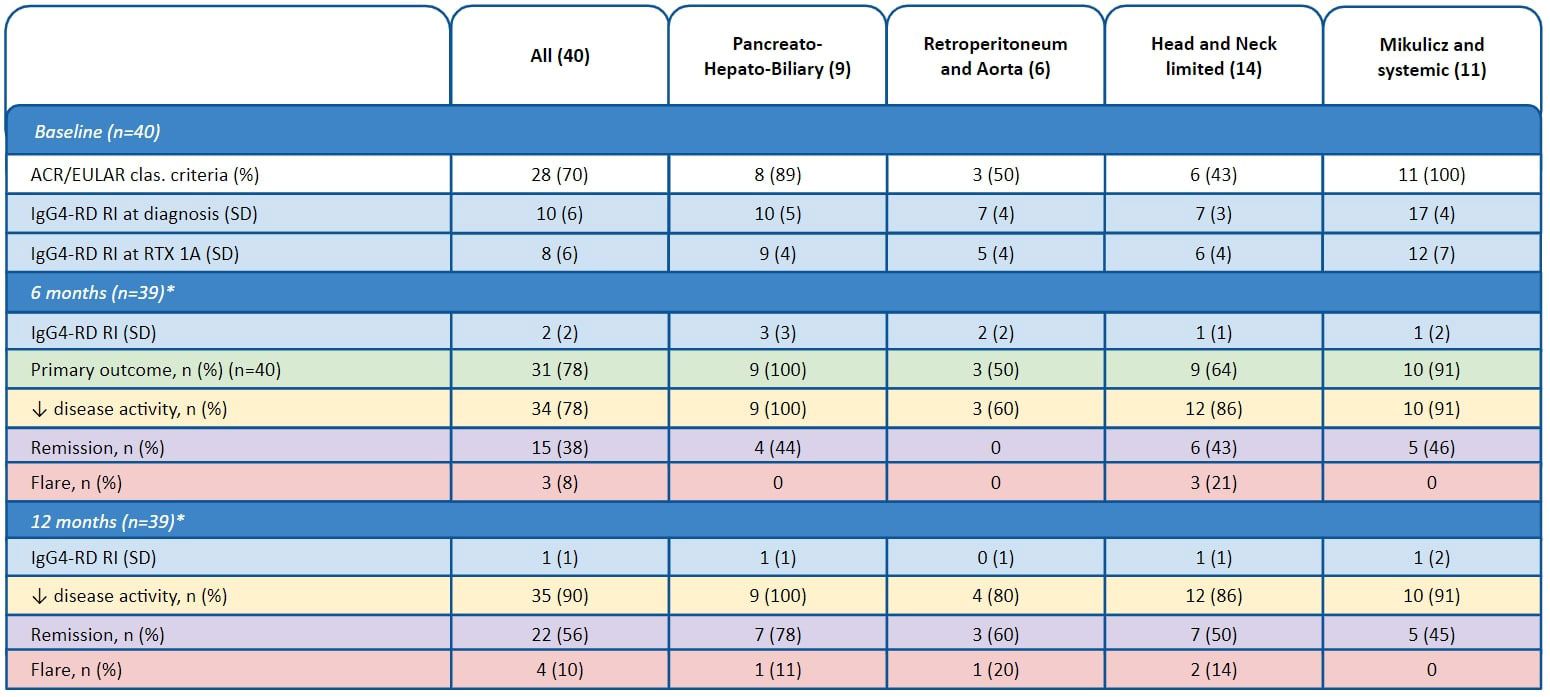
Results: We included 40 patients, of which 30 (75%) were male and 35 (88%) Caucasian. Mean age and disease duration at time of first RTX infusion was 58 and 3 years, respectively. Seventeen of the 40 patients (43%) received RTX as add-on therapy (following GC for > 3 months), while 13 (33%) received RTX as upfront combination therapy with GC, and 10 (25%) received RTX as upfront monotherapy. All 40 patients received an infusion of 1000 mg RTX at study baseline (dose 1A at week 0) and 39 of these 40 patients (98%) received a second RTX infusion (dose 1B) at week 2. Additional infusions of 500-1000 mg RTX were administered at weeks 26 (dose 2A) and 28 (dose 2B) in 24 (60%) and 7 (18%) patients, respectively. The composite primary endpoint was met by 31/40 patients (78%). Reduced disease activity at 6 and 12 months were seen in 34 (87%) and 35 (90%) patients, respectively. Fifteen patients (38%) were in remission at 6 months, and 22 (56%) were in remission at 12 months. “Retroperitoneum and Aorta” showed lowest response rates, while “Head and Neck-Limited” had the highest rate of flares (Table 1). Mild infusion reaction occurred in 8 (20%) patients. Hypogammaglobulinemia was observed in 4 (10%). Infection requiring hospitalization occurred in 6 (15%), including one fatal infection which was the only death in the study period.
Conclusion: In our observational study, RTX appears safe and effective in IgG4-RD, with the highest response in patients with Pancreato-Hepato-Biliary phenotype. Relatively low remission rates across all phenotypes indicate an unmet need for improved treatment
To cite this abstract in AMA style:
Vikse J, Midtvedt O, Molberg O, Fevang B, Palm O, Garen T, Norheim K, Bakland G, Wallenius M, Hoffmann-Vold A. Rituximab in IgG4-Related Diseae: An Open-Label Non-Randomized Observational Study [abstract]. Arthritis Rheumatol. 2022; 74 (suppl 9). https://acrabstracts.org/abstract/rituximab-in-igg4-related-diseae-an-open-label-non-randomized-observational-study/. Accessed .« Back to ACR Convergence 2022
ACR Meeting Abstracts - https://acrabstracts.org/abstract/rituximab-in-igg4-related-diseae-an-open-label-non-randomized-observational-study/