Session Information
Session Type: Poster Session A
Session Time: 1:00PM-3:00PM
Background/Purpose: Alternate-day glucocorticoid (GC) treatment is an effective treatment option that can reduce GC-associated adverse events in patients with rheumatoid arthritis and other autoimmune rheumatic diseases. The treatment strategy has not been evaluated in patients with immunoglobulin G4-related disease (IgG4-RD). We investigated the safety and efficacy of alternate-day GC therapy in patients with IgG4-RD.
Methods: Medical records of patients with IgG4-RD who were followed for at least one year at a tertiary-care referral center from 2004 to 2020 were reviewed. Patients who fulfilled comprehensive IgG4-RD diagnostic criteria were divided into alternate-day or daily GC treatment groups based on their treatment protocol. Propensity scores for the possibility of adopting alternate-day GC therapy were calculated. The influences of alternate-day GC therapy on glucocorticoid toxicity index (GTI) were evaluated at months 6, 12, 18, and 24 after treatment initiation using multivariable linear analysis with adjustments for cumulative GC doses until each assessment point and propensity scores. Kaplan-Meier curves and Cox proportional hazard models were used to assess the efficacy of alternate-day GC therapy for disease control.
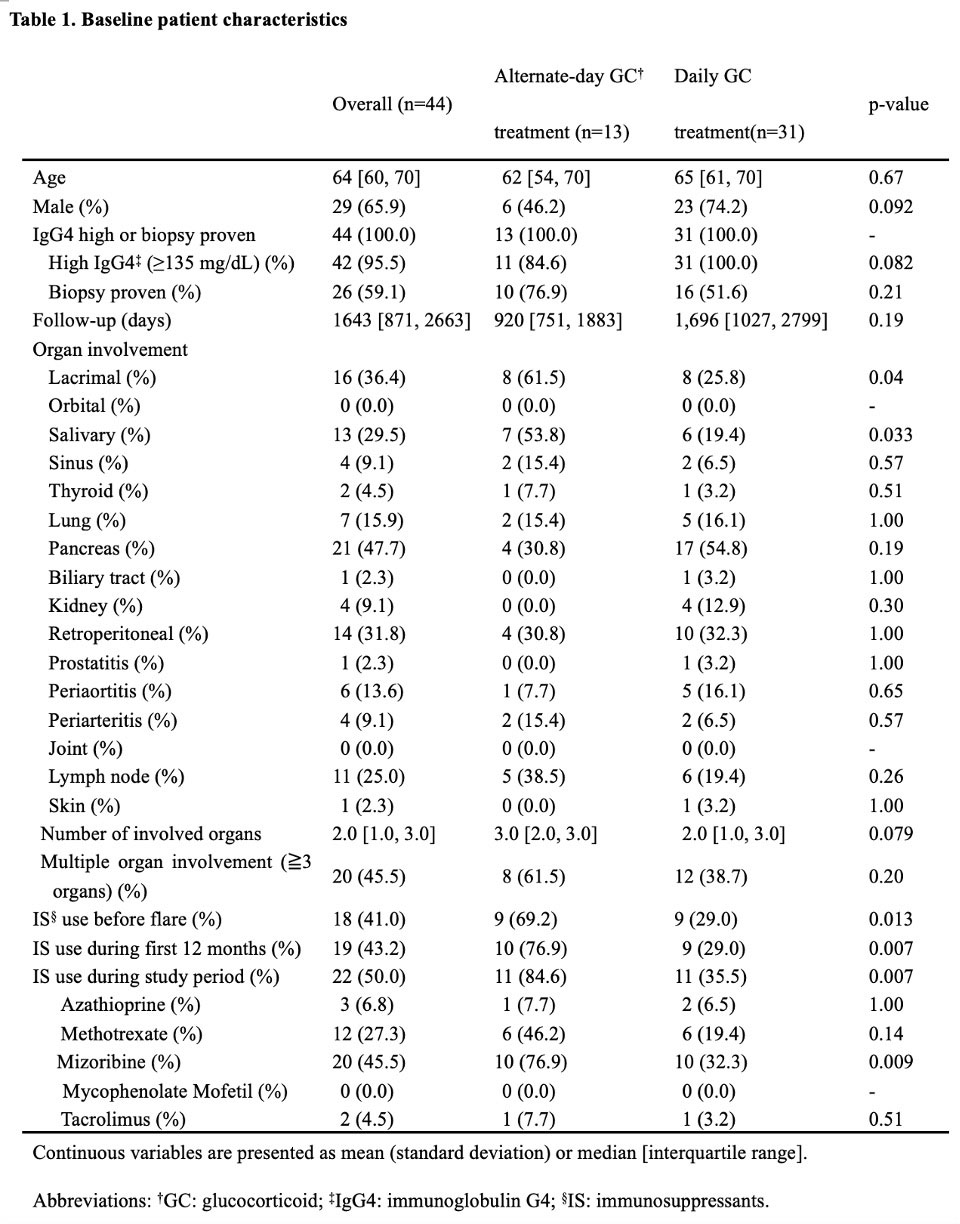
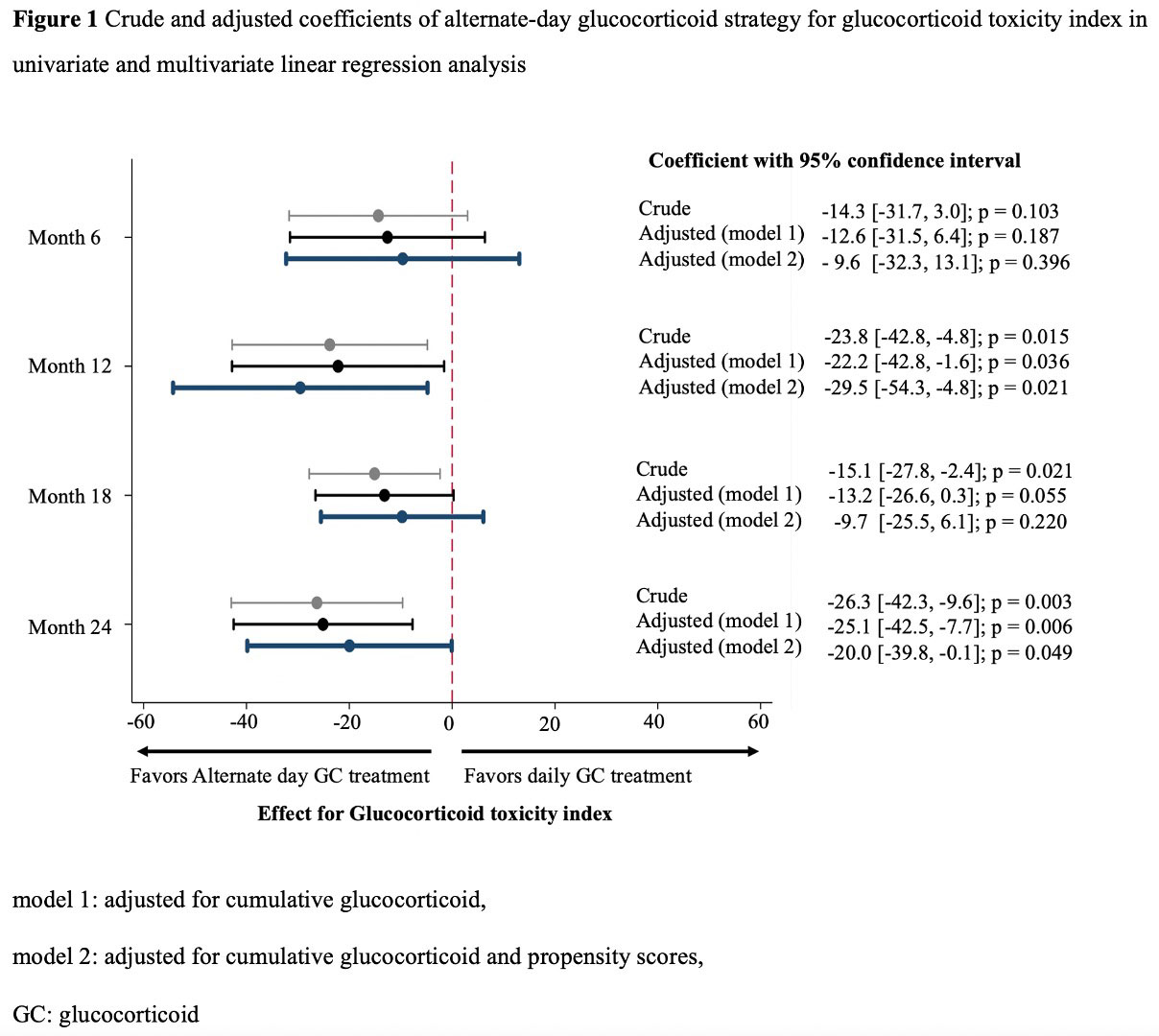
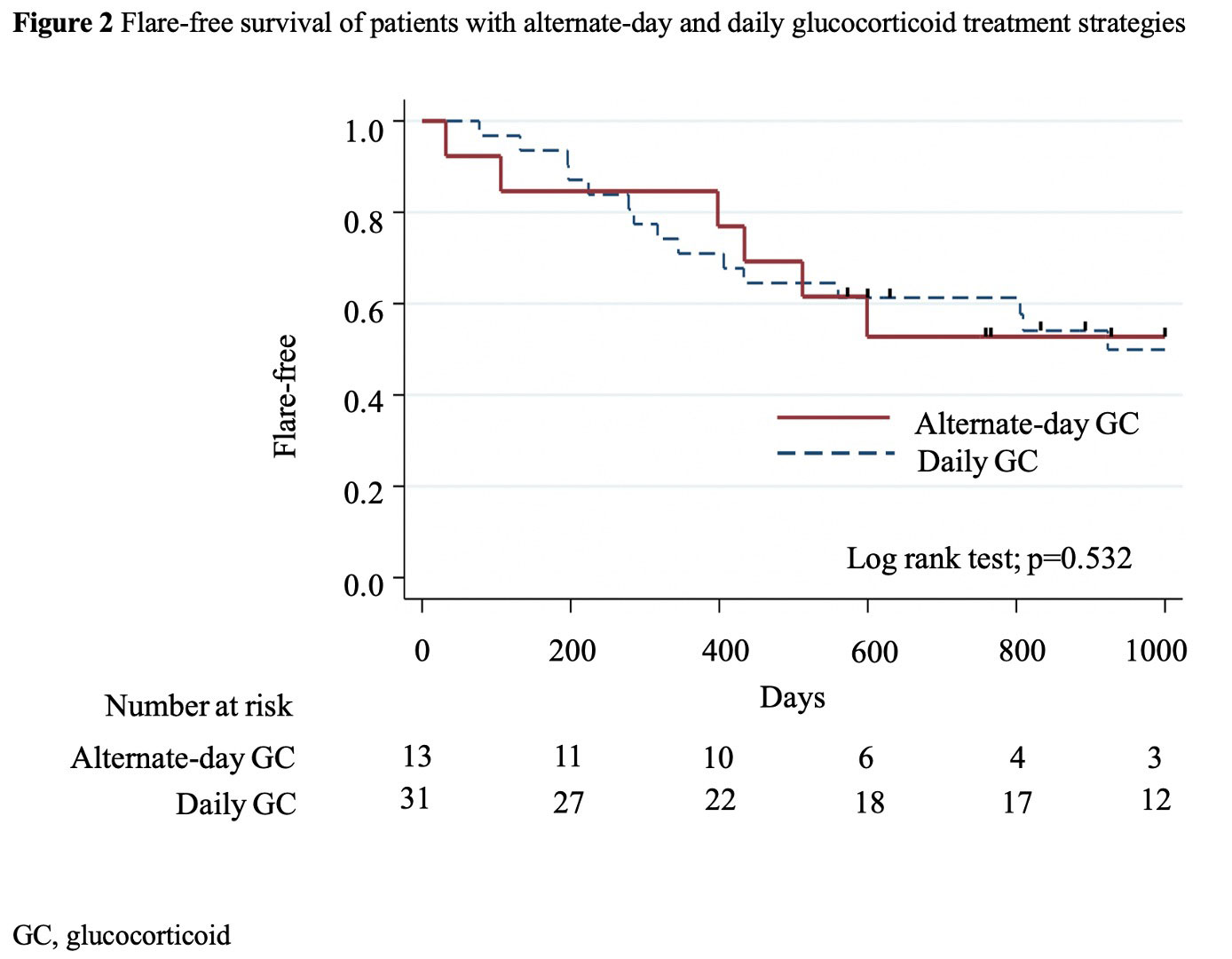
Results: Among the 67 patients with IgG4-RD , eligible patients with alternate-day (n=13) and daily (n=31) GC treatments were analyzed. The median (IQR) age was 64 (60–70) years, 29 (65.9%) were male patients, 26 (59.1%) patients had positive biopsy results, and the median follow-up period was 1,643 (871–2,663) days. Significantly more patients with alternate-day GC treatment used concomitant immunosuppressants before flare of IgG4-RD (9 [69.2%] vs. 9 [29.0%]; p=0.013) (Table 1). The alternate-day GC strategy significantly lowered the GTI score after adjusting for cumulative GC dose and propensity scores at months 12 and 24 (adjusted coefficient [95%CI]: -29.5 [-54.3, -4.8], p=0.021 at 12 months; -20.0 [-39.8, -0.1], p=0.049 at 24 months) (Figure 1). Serious infections were numerically less frequent in the alternate-day group (incidence rate ratio [95% CI]: 0.45 [0.05, 3.63], p=0.451). There was only one case that failed to respond to alternate-day GC in remission induction treatment. Kaplan-Meier curve showed no significant differences in the flare of IgG4RD (p =0.532) (Figure 2). The propensity score-adjusted hazard ratio of alternate-day GC treatment for disease flares was not significant (1.55 [0.53, 4.51], p=0.425).
Conclusion: Alternate-day treatment strategy significantly reduced GC-related adverse events even after adjusted for cumulative GC doses, without a significant increase in disease flares particularly when combined with immunosuppressants. Alternate-day GC treatment can be a feasible option for patients with IgG4-RD.
To cite this abstract in AMA style:
Fukui S, Nakai T, Kawaai S, Ikeda Y, Suda M, Nomura A, Tamaki H, Kishimoto M, Ohde S, Okada M. Advantages of Alternate-Day Glucocorticoid Treatment Strategy for IgG4-Related Disease: A Preliminary Retrospective Study ID: 1287015 [abstract]. Arthritis Rheumatol. 2022; 74 (suppl 9). https://acrabstracts.org/abstract/advantages-of-alternate-day-glucocorticoid-treatment-strategy-for-igg4-related-disease-a-preliminary-retrospective-study-id-1287015/. Accessed .« Back to ACR Convergence 2022
ACR Meeting Abstracts - https://acrabstracts.org/abstract/advantages-of-alternate-day-glucocorticoid-treatment-strategy-for-igg4-related-disease-a-preliminary-retrospective-study-id-1287015/