Session Information
Date: Tuesday, November 9, 2021
Title: Spondyloarthritis Including PsA – Treatment Poster III: Psoriatic Arthritis II (1801–1835)
Session Type: Poster Session D
Session Time: 8:30AM-10:30AM
Background/Purpose: Secukinumab has demonstrated to be efficacious for both psoriatic arthritis (PsA) and ankylosing spondylitis (AS): PASI, ACR and ASAS magnitudes of response have generally been higher in the anti-TNF-naïve population. However, real world data on effectiveness in quality of life (QoL) and function are missing.
To analyze the differences of secukinumab therapy (effectiveness in disease activity and QoL, persistence and safety profile) in biologic-naïve versus biologic-experienced patients.
Methods: Multicenter, observational study of PsA and AS patients using real world anonymous patient-level data from the Portuguese national register database – Reuma.pt. We analyzed data at baseline and after 3, 6 and 12 months after secukinumab initiation, between January 2017 and January 2021. We collected data on sociodemographic characteristics and safety (discontinuation and adverse events); effectiveness [ACR and ASAS response, patient global assessment (PtGA), physician global assessment (PhGA), C-reactive protein (CRP), erythrocyte sedimentation rate (ESR), BASDAI, ASDAS-CRP, DAPSA and DAS28 4 V]; function and QoL [ASQoL (Ankylosing Spondylitis QoL questionnaire), EQ-5D (Euro-QoL-5 dimensions), FACIT-F (Functional Assessment of Chronic Illness Therapy-Fatigue); HADS (Hospital Anxiety and Depression Scale); HAQ (Health Assessment Questionnaire); BASFI and SF-36 (Health Questionnaire Short-Form 36)].
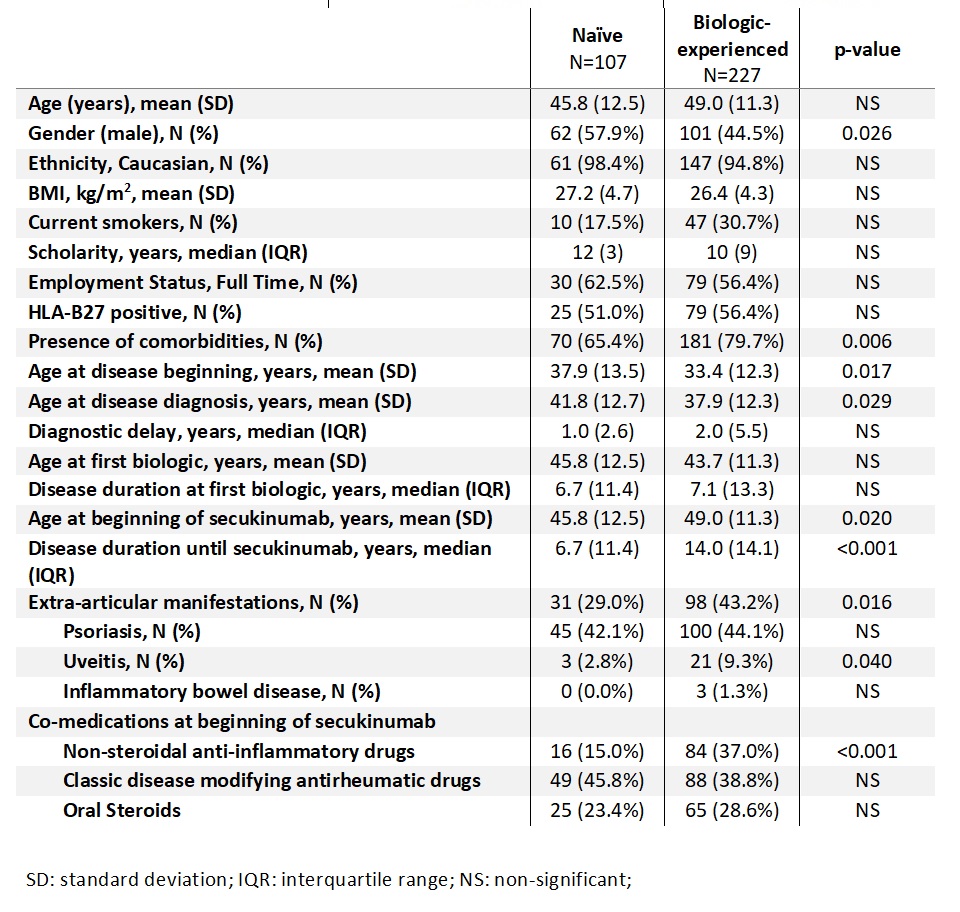
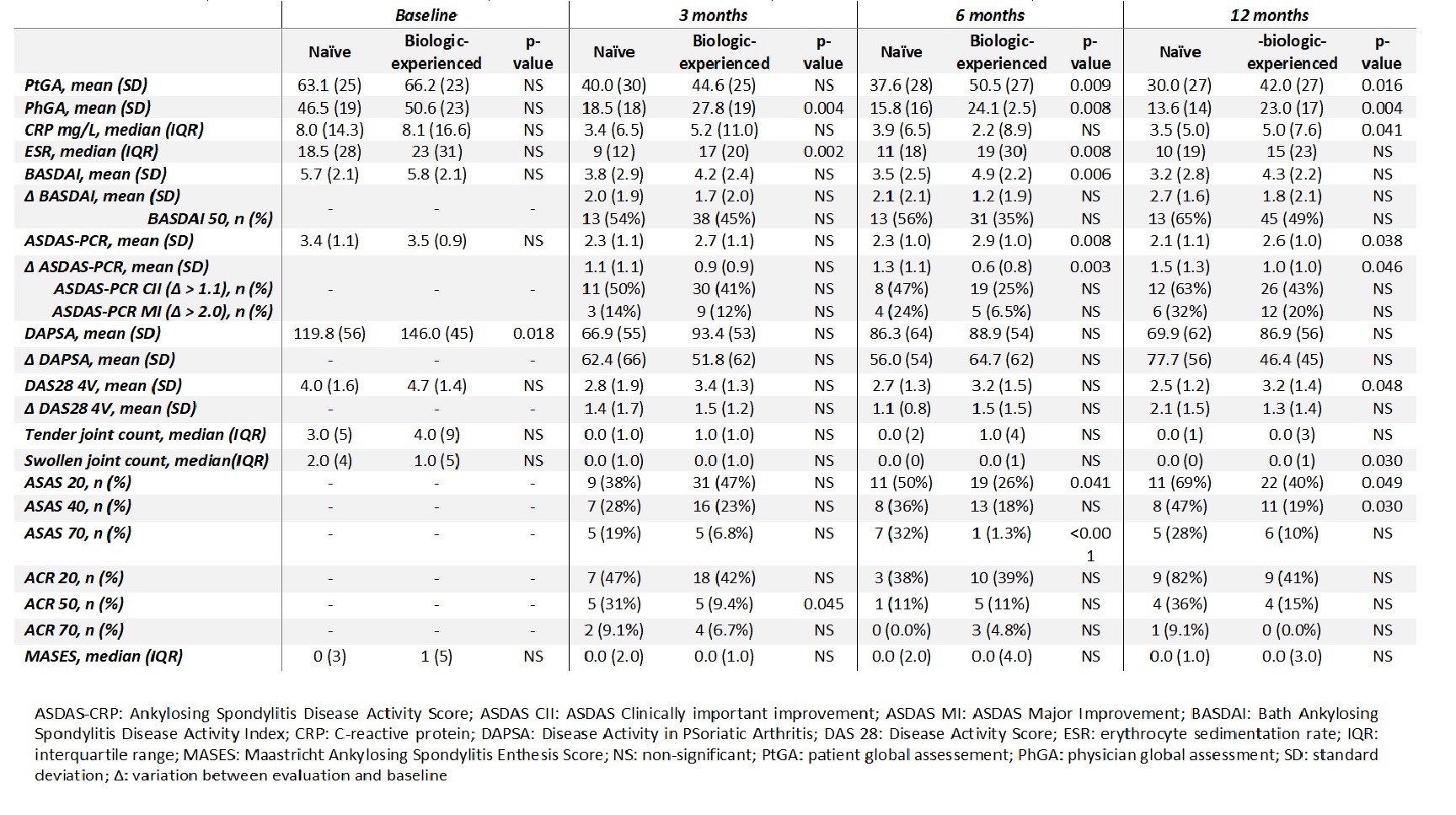
Results: We included 334 patients (166 with PsA and 168 with AS), 68% were biologic-experienced. Differences between biologic naïve and biologic-experienced patients’ clinical characteristics are shown in Table 1. When we analyzed disease activity, we found that PhGA (p=0.004) and ESR (p=0.002) were significantly lower in naïve patients and more naïve patients reached ACR50 response (p=0.045) at 3 months. At 6 months evaluation, in addition to PhGA (p=0.008) and ESR (p=0.008) that remained significantly lower in naïve patients, PtGA (p=0.009), ASDAS-PCR (p=0.008) and BASDAI (p=0.006) were also lower in naïve patients. In the last evaluation, at 12 months, besides the variables described, also DAS 28 4V was lower in naïve patients (p=0.048) (Table 2).
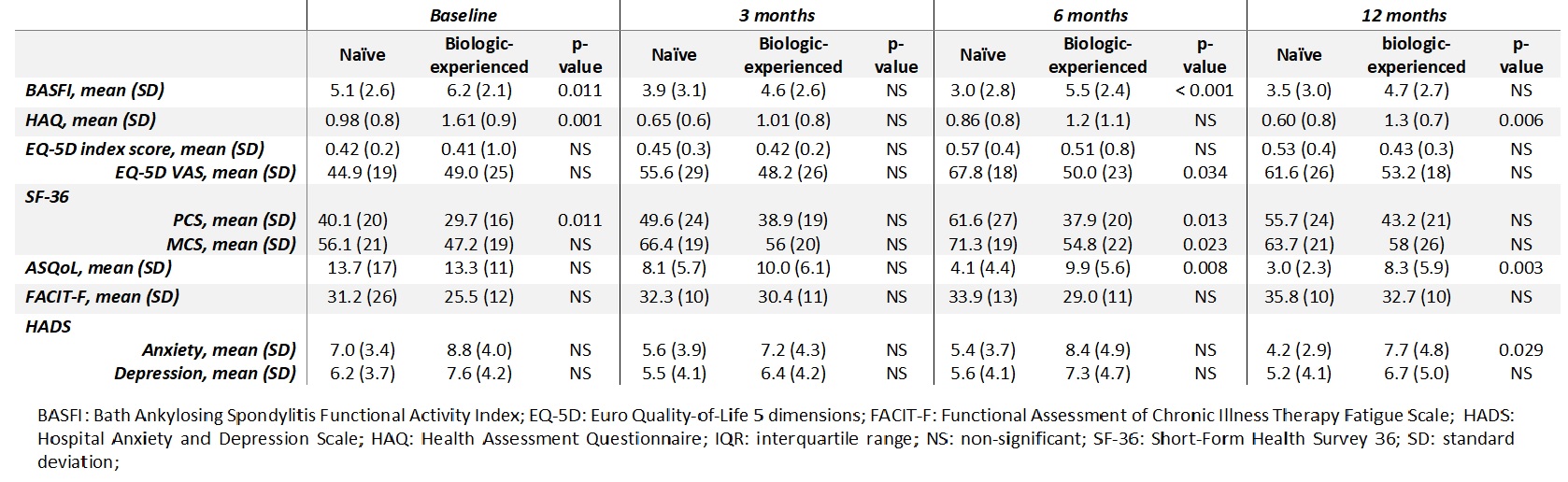
Regarding QoL and function, at 6 months, BASFI (p< 0.001), EQ5D-VAS (p=0.034), SF-36 scores, physical (p=0.013) and mental (p=0.023) domains and ASQoL (p=0.008) were significantly better in naïve patients. After a year of follow-up (12 months evaluation) naïve patients had a better health status (HAQ and ASQoL) (p=0.006 and p=0.003 respectively) and biologic-experienced patients had more anxiety (HADS, p=0.029) (Table 3).
Regarding, drug persistence, although not statistically significant, biologic-experienced patients were more prone to discontinue the drug (18.5% and 11.2%, respectively, p=0.091)
Regarding safety, there were no differences in the number or type of severe (naïve patients: 3/107 vs biologic-experienced: 9/227) or mild (naïve patients: 4/107 vs biologic-experienced: 7/227) adverse effects.
Conclusion: Secukinumab treatment showed a larger magnitude of response (disease activity, function and QoL) in the biologic-naïve population, both in PsA and AS patients. No differences on safety profile were found.
To cite this abstract in AMA style:
Azevedo S, Tavares-Costa J, Laires P, Garcia S, Nero P, Pestana J, Martins-Martinho J, Bento Silva A, Rodrigues J, Sequeira G, Costa E, Cunha Santos F, Furtado C, Santos B, Raposo A, Saraiva L, Samões B, Barcelos F, José Santos M, Santos H. Secukinumab Therapy in Biologic-naïve vs. Biologic-experienced Patients: Real-world Effectiveness, Persistence and Safety Results from the Rheumatic Diseases Portuguese Registry [abstract]. Arthritis Rheumatol. 2021; 73 (suppl 9). https://acrabstracts.org/abstract/secukinumab-therapy-in-biologic-naive-vs-biologic-experienced-patients-real-world-effectiveness-persistence-and-safety-results-from-the-rheumatic-diseases-portuguese-registry/. Accessed .« Back to ACR Convergence 2021
ACR Meeting Abstracts - https://acrabstracts.org/abstract/secukinumab-therapy-in-biologic-naive-vs-biologic-experienced-patients-real-world-effectiveness-persistence-and-safety-results-from-the-rheumatic-diseases-portuguese-registry/