Session Information
Date: Tuesday, November 9, 2021
Title: RA – Treatments Poster III: RA Treatments & Their Safety (1674–1710)
Session Type: Poster Session D
Session Time: 8:30AM-10:30AM
Background/Purpose: Baricitinib, an oral selective inhibitor of Janus kinase (JAK) 1 and 2, improved signs and symptoms of rheumatoid arthritis(RA).We analyze efficacy and safety of baricitinib in real-world data.
Methods: Cases were recruited to SHin-yokohama Arthritis REgister (SHARE) between 2015 and 2020 (n=3,961). 154 Patients were diagnosed according to ACR/EULAR 2010 classification criteria and treated with baricitinib over 15 months. 32 cases fulfilled EULAR definition for difficult-to-treat RA (D2T-RA). In 154 (Male25, Female129 cases, RA duration 11.4+/-7.8years) cases, Clinical Disease Activity Index (CDAI), Health Assessment Questionnaire-Disability Index (HAQ-DI), anti-CCP2 and other clinical parameters were analyzed. They were arrayed based on previous treatments as b/tsDMARD-naïve and b/tsDMARD-insufficient responders (IR) after the failure or intolerance to bDMARDs. Tapering MTX dose was also analyzed in these two groups.
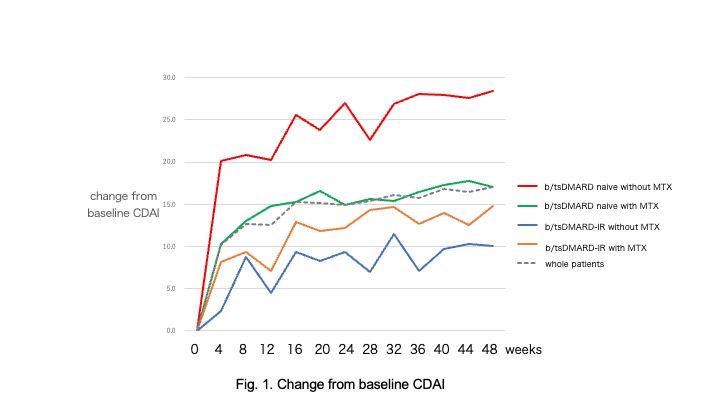
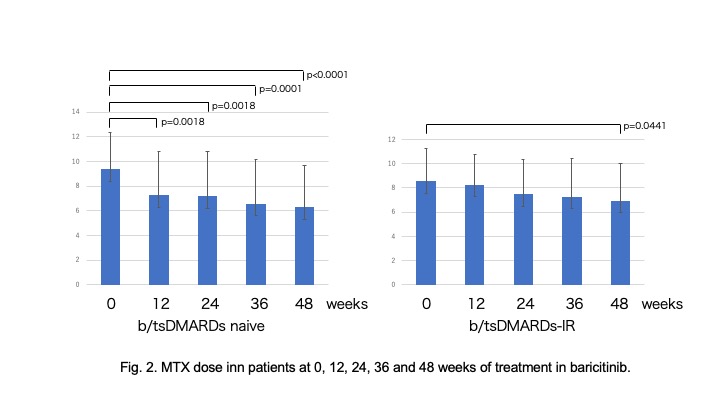
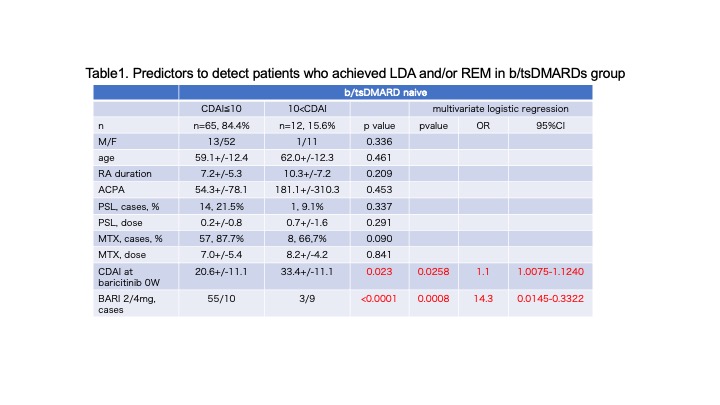
Results: 79 (51.3%) b/tsDMARDs naïve and 75(48.7%)b/tsDMARDs-IR patients were enrolled. There were no differences in RA duration time (11.4+/-7.8 vs. 12.9+/-8.3), anti-CCP2 positive(ave.242.6+/-158.9), and CDAI(20.2+/-12.4 vs. 17.8+/-11.3) at the beginning of baricitinib. Baricitinib withdrawal for inefficacy showed no difference between b/tsDMARDs naive and b/tsDMARDs-IR patients in RA with/without MTX (logrank p=0.8589). In b/tsDMARDs naïve group, 45(59.5%) patients were achieved LDA and 18 (22.8%) were acheived remission at 12 weeks. In b/tsDMARDs-IR group, 35(46.7%) patients were achieved LDA and 11(14.7%) patients were achieved remission. b/tsDMARDs naïve group showed more changes from baseline CDAI. In b/tsDMARDs group, predictors to detect patients who achieved LDA and/or remission were lower CDAI(20.6+/-11.1 vs. 33.4+/-11.1) and baricitinib 2mg/day in multivariate logistic regression( OR 1.1 and 14.3, 95%CI 1.0075-1.1240 and 0.0145-0.3322, p=0.023 and p< 0.0001, respectively). In A significant reduction in the dose of MTX was seen at 48 weeks in both b/tsDMARDs naïve group and b/tsDMARDs-IR group (p< 0.0001 vs. p=0.0441). 11(13.9%) parients were discontinued baricitinib due to herpes zoster (8.1/100PY). There were no thrombotic event in our cohort.
Conclusion: Our data confirm the efficacy and safety profiles of baricitinib in RA. It also showed baricitinib 2mg/day was effective in b/tsDMARD naïve patients.
To cite this abstract in AMA style:
YAMASAKI M. Efficacy and Safety of Baricitinib in B/tsDMARDs Naive and B/tsDMARDs-IR Patients with Rheumatoid Arthritis [abstract]. Arthritis Rheumatol. 2021; 73 (suppl 9). https://acrabstracts.org/abstract/efficacy-and-safety-of-baricitinib-in-b-tsdmards-naive-and-b-tsdmards-ir-patients-with-rheumatoid-arthritis/. Accessed .« Back to ACR Convergence 2021
ACR Meeting Abstracts - https://acrabstracts.org/abstract/efficacy-and-safety-of-baricitinib-in-b-tsdmards-naive-and-b-tsdmards-ir-patients-with-rheumatoid-arthritis/