Session Information
Session Type: Poster Session D
Session Time: 8:30AM-10:30AM
Background/Purpose: The development and duration of humoral immunity after SARS-CoV-2 natural infection remains of interest. For the general population, available data suggest a robust immune response, able to protect against reinfection for at least 6-8 months. As for the subgroup of patients with rheumatic and musculoskeletal diseases (RMDs), information is lacking. Considering that immunosuppression might preclude an adequate anti-viral response, we aimed to assess the rate of seroconversion after natural infection in patients with RMDs and to find factors that may influence antibody response.
Methods: Multicenter observational study of patients with RMDs prospectively-followed in the Rheumatic Diseases Portuguese Register – Reuma.pt –in the first 6 months of the pandemic in Portugal – March to September 2020. We included all patients with inflammatory and non-inflammatory RMDs with confirmed (PCR-positive) or suspected COVID-19. Patients were asked to collect a blood sample for antibody testing against SARS-CoV-2 at least 3 months after the resolution of infection. All samples were processed in a single center. IgG antibodies recognizing the SARS-CoV-2 receptor-binding domain (RBD) were quantified using ELISA. Seroconversion was assumed for any titer ≥1:50.
Results: Out of 179 included patients, 79 (44%) performed antibody testing. Of these, 65 (82%) had inflammatory RMDs and 14 (18%) had non-inflammatory RMDs – described in Table 1. Blood samples were collected between days 89 and 331 (median time 237, IQR 125 days) after symptom onset or positive PCR test (if asymptomatic). Seventy (89%) patients had positive IgG antibodies, with a geometric mean titer of 1/1508±4.075 (min 1/100- max 1/25600).
No differences were seen in the seroconversion rate between patients with and without inflammatory RMDs. Disease activity status at the time of the infection also did not influence seroconversion. Although DMARD therapy didn’t influence seropositivity, the proportion of patients under TNF inhibitors (TNFi) was numerically higher in patients who did not develop IgG antibodies (33.3% vs 8.6%, p=0.062). Of note, all patients treated with corticosteroids (N=30) and rituximab (N=2) developed antibodies. There was no correlation between sample timing and RBD IgG titers. On multivariate analysis, treatment with TNFi (OR 0.13, 95%CI: 0.02-0.91, p=0.041), and symptomatic COVID-19 (OR 15.1, 95%CI 2.33-98.48; p=0.004) were the only variables independently associated with serological response (Table 2).
Conclusion: Most patients with rheumatic diseases developed IgG antibodies against SARS-CoV-2, with medium-to-high titers detected between 3 to 11 months after natural infection. In this population, treatment with TNFi decreased the odds of seroconversion while symptomatic COVID-19 was associated with a higher likelihood of developing a humoral immune response.
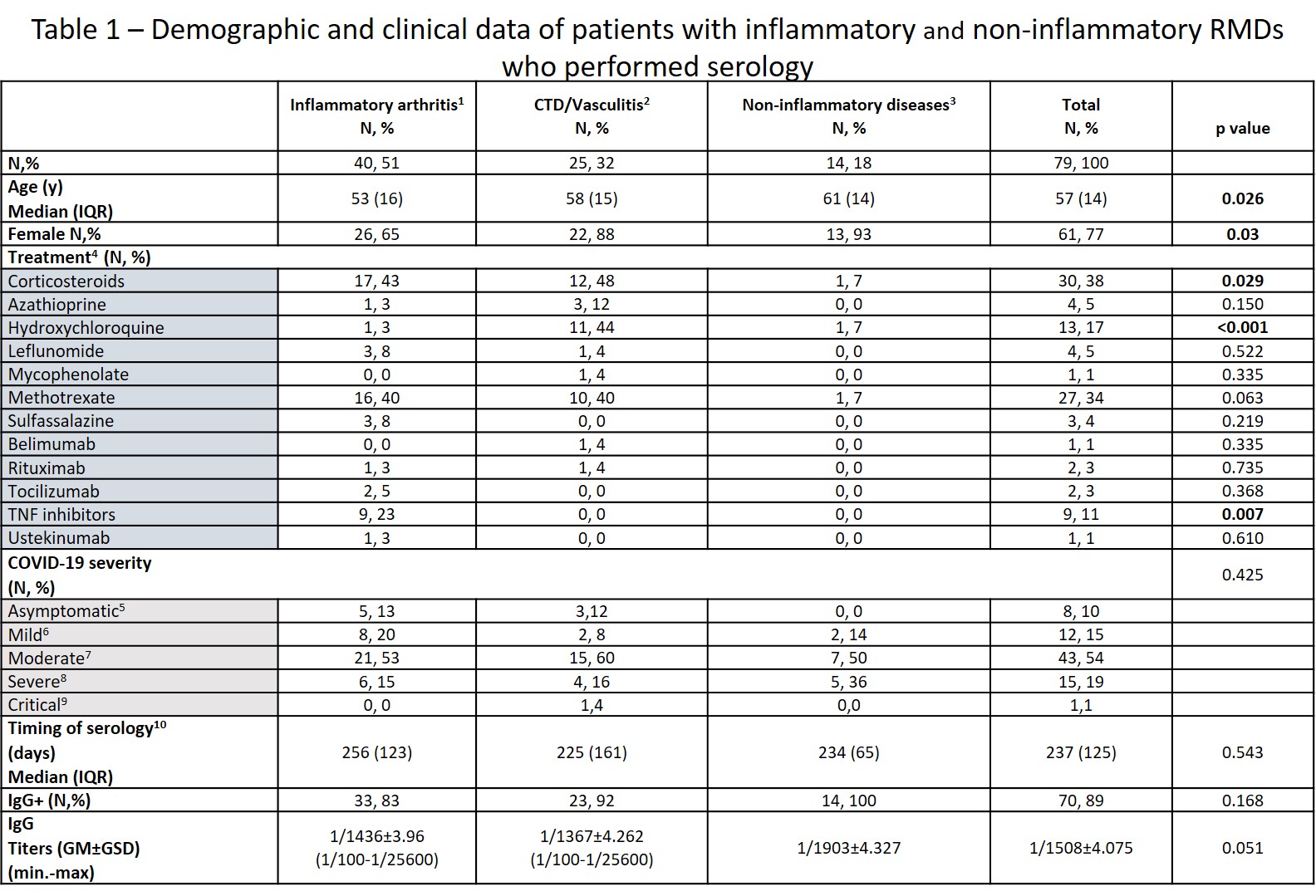 1 – Includes rheumatoid arthritis, psoriatic arthritis (PsA), spondyloarthritis other than PsA, RS3PE, undifferentiated arthritis and microcrystalline arthritis, adult onset Still disease. 2 – Includes systemic lupus erythematosus, undifferentiated connective tissue disease, mixed connective tissue disease, systemic sclerosis, Sjögren syndrome, giant cell arteritis, Behçet disease. 3 – Fibromyalgia, osteoarthritis, osteoporosis, Paget bone disease. 4 – treatment before infection; only DMARDs and corticosteroids were considered; 5 – reference for statistical analysis; 6 – symptomatic disease without evidence of pneumonia; 7 – clinical signs of pneumonia but room-air SpO2 ≥ 90%; 8 – hypoxemic pneumonia and/or need for hospitalization; 9 – requiring admission to intensive care unit or death. 10 – relative to symptom onset or first positive PCR test if asymptomatic. CTD – connective tissue diseases; GM: geometric mean; GSD: geometric SD factor; y: years.
1 – Includes rheumatoid arthritis, psoriatic arthritis (PsA), spondyloarthritis other than PsA, RS3PE, undifferentiated arthritis and microcrystalline arthritis, adult onset Still disease. 2 – Includes systemic lupus erythematosus, undifferentiated connective tissue disease, mixed connective tissue disease, systemic sclerosis, Sjögren syndrome, giant cell arteritis, Behçet disease. 3 – Fibromyalgia, osteoarthritis, osteoporosis, Paget bone disease. 4 – treatment before infection; only DMARDs and corticosteroids were considered; 5 – reference for statistical analysis; 6 – symptomatic disease without evidence of pneumonia; 7 – clinical signs of pneumonia but room-air SpO2 ≥ 90%; 8 – hypoxemic pneumonia and/or need for hospitalization; 9 – requiring admission to intensive care unit or death. 10 – relative to symptom onset or first positive PCR test if asymptomatic. CTD – connective tissue diseases; GM: geometric mean; GSD: geometric SD factor; y: years.
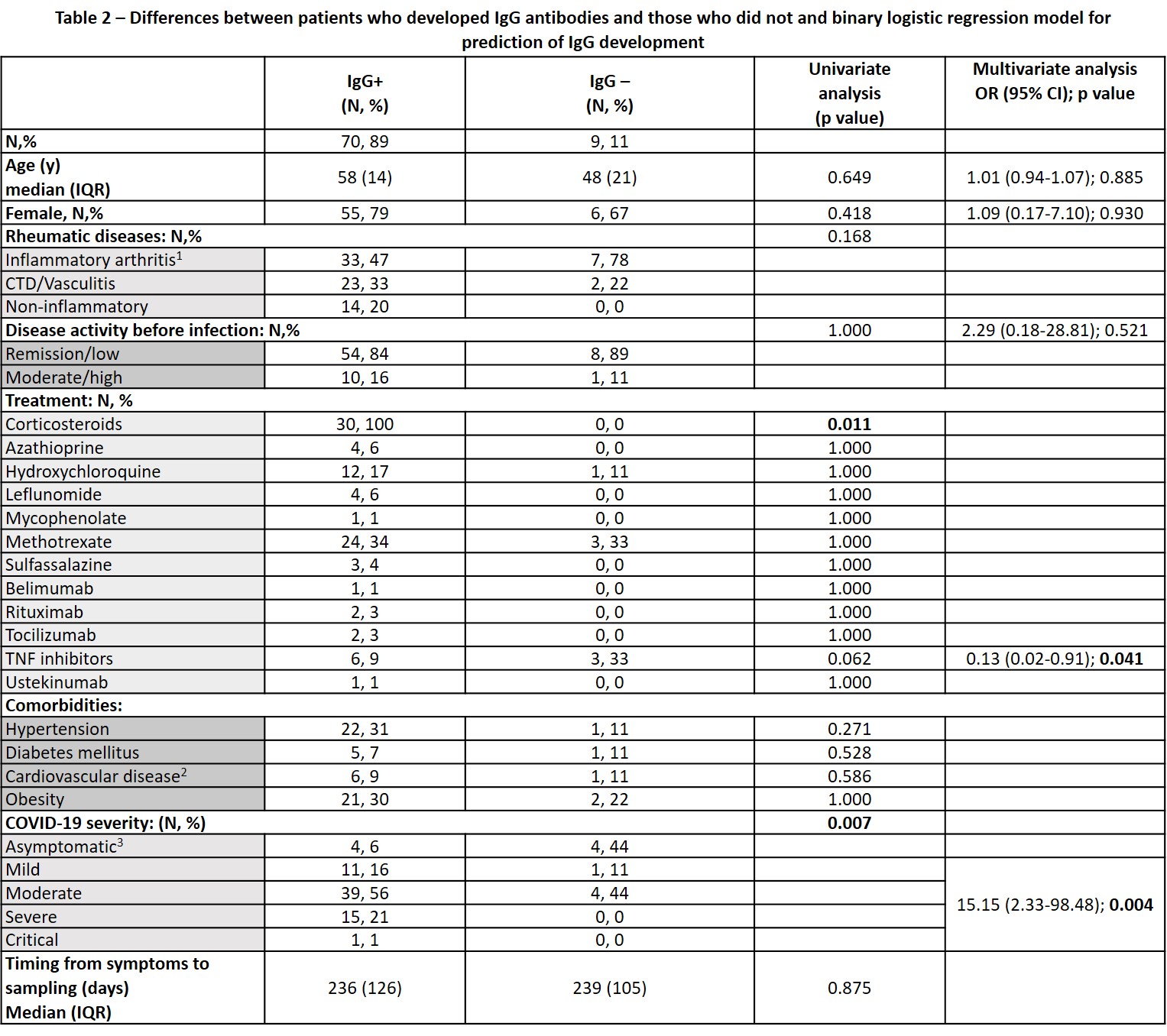 1 –reference for statistical analysis; 2 – includes personal history of heart failure and/or ischemic cardiopathy. 3 – reference for statistical analysis. CTD: connective tissue diseases.
1 –reference for statistical analysis; 2 – includes personal history of heart failure and/or ischemic cardiopathy. 3 – reference for statistical analysis. CTD: connective tissue diseases.
To cite this abstract in AMA style:
Cruz-Machado A, Carvalho Barreira S, Veldhoen M, Bandeira M, Duarte C, Rato M, Fernandes B, Garcia S, Pinheiro F, Bernardes M, Madeira N, Miguel C, Torres R, Bento Silva A, Mazeda C, Cunha Santos F, Sousa M, Parente H, José Santos M, Fonseca J, Romão V. Antibody Response After SARS-CoV-2 Infection in Patients with Rheumatic Diseases: A Multicenter, Nationwide Study [abstract]. Arthritis Rheumatol. 2021; 73 (suppl 9). https://acrabstracts.org/abstract/antibody-response-after-sars-cov-2-infection-in-patients-with-rheumatic-diseases-a-multicenter-nationwide-study/. Accessed .« Back to ACR Convergence 2021
ACR Meeting Abstracts - https://acrabstracts.org/abstract/antibody-response-after-sars-cov-2-infection-in-patients-with-rheumatic-diseases-a-multicenter-nationwide-study/
