Session Information
Session Type: Poster Session D
Session Time: 8:30AM-10:30AM
Background/Purpose: Rituximab is an anti-CD20 antibody with therapeutic use in myositis. However, given its B cell depleting mechanism, there is concern regarding its association with hypogammaglobinemia and consequent risk of infection in myositis. We therefore aimed to perform a comprehensive analysis of the effect of rituximab on IgG levels of DM/PM/JDM patients and the association of IgG levels with infections using the Rituximab in Myositis (RIM) study data.
Methods: We retrospectively analyzed data collected in the RIM Trial, which included a rituximab early (RE) and late (RL) group with rituximab administered at weeks 0/1 and at week 8/9, respectively (Figure 1). Serum IgG levels were measured as the ratio of absolute IgG level divided by the lower limit of normal (LLN) for the reference lab. Data were compared for the overall, RL, and RE groups, with/without stratification by disease states that included DM, PM and JDM. IgG levels were compared using a paired t-test. Frequency and duration of abnormally low IgG levels was also evaluated. Associations between the number of infections and IgG levels as well as baseline immunosuppressive drugs were determined using spearman correlation.
Results: A total of 194 patients (72 DM, 74 PM, 48 JDM) meeting Bohan & Peter criteria were included in this study; with mean age 41 years, 73% female and 71% Caucasian. There was a small, but significant decrease in mean IgG levels (1.9 vs 1.7 xLLN) seen from baseline to week 8, significant only in RE (Early: 2.0 vs. 1.7, p=0.005; Late: 1.8 vs. 1.6, p=0.10) (Figure 2). Nadir IgG levels showed a small but significant decline from baseline levels in both RE and RL; however, end of trial levels were significantly decreased only in RE. Similar trends were seen in subgroup analyses of adult DM, PM, and JDM; except that end of trial levels were only significantly lower from baseline in DM (p< 0.0001), with a non-significant decline in PM (p=0.110 and JDM (p=0.11).
Despite the small decline in IgG levels, most patients (77%) had IgG levels that remained normal. Amongst 45 (23%) patients with IgG levels below LLN at any time, there were 3 trends: (a) patients with IgG levels below LLN at baseline (n=18); (b) patients with transient decline of IgG levels below LLN (n=14); (c) patients who developed long term decline of IgG levels below LLN (n=13).
Overall, 56.2% patients had infections during the study, with the average number of infections being 2.2 (Figure 3). There was no association between IgG level and number of infections anytime during the trial except in the DM subgroup, where more infections were seen in patients with IgG levels below LLN at baseline (median # infections: 4 vs 2, p=0.027). There was no correlation between number of immunosuppressant medications or baseline steroid dose and number of infections.
Conclusion: Rituximab led to a modest, but statistically significant decline in IgG in myositis patients of unclear clinical significance, without clear association between IgG levels and infection except in the DM subgroup with IgG levels below LLN at baseline. These data should provide additional evidence supporting the safety of rituximab in myositis, where most patients’ IgG levels remained normal or declined only transiently.
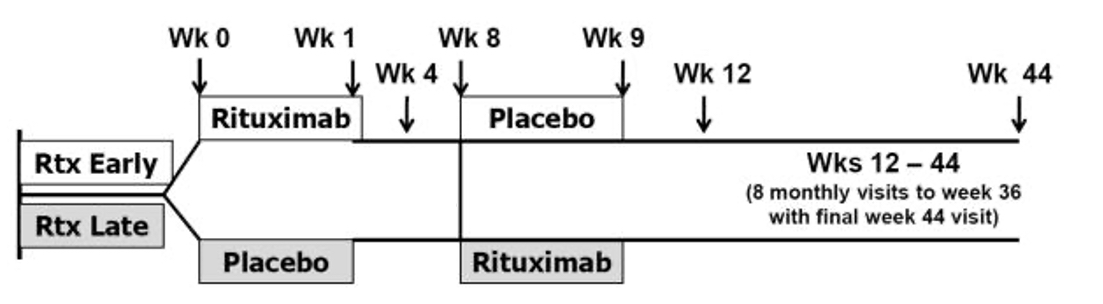 Study design of Rituximab in Myositis (RIM) study
Study design of Rituximab in Myositis (RIM) study
 Mean IgG/LLN levels for baseline through week 8 for rituximab late vs rituximab early
Mean IgG/LLN levels for baseline through week 8 for rituximab late vs rituximab early
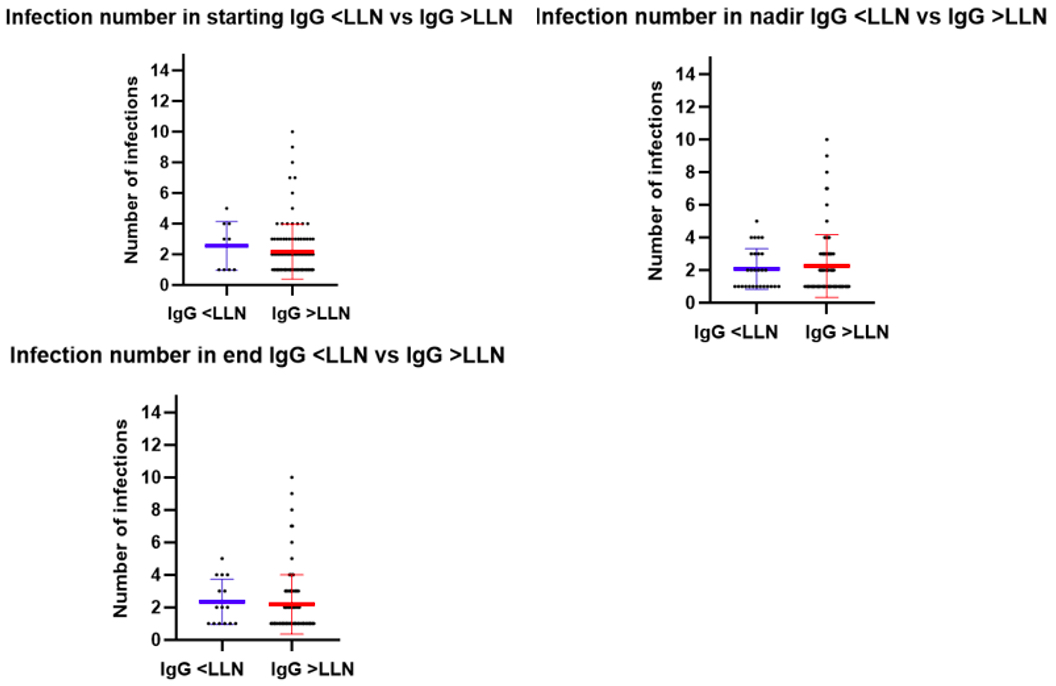 Infection number by LLN status at baseline, nadir and ending level. Note, plotted lines include mean in middle, SD at top/bottom
Infection number by LLN status at baseline, nadir and ending level. Note, plotted lines include mean in middle, SD at top/bottom
To cite this abstract in AMA style:
Macklin M, Oddis C, Moghadam-Kia S, Ascherman D, Aggarwal R. Effect of Rituximab on IgG Levels and Associated Infection Risk in Myositis [abstract]. Arthritis Rheumatol. 2021; 73 (suppl 9). https://acrabstracts.org/abstract/effect-of-rituximab-on-igg-levels-and-associated-infection-risk-in-myositis/. Accessed .« Back to ACR Convergence 2021
ACR Meeting Abstracts - https://acrabstracts.org/abstract/effect-of-rituximab-on-igg-levels-and-associated-infection-risk-in-myositis/
