Session Information
Date: Monday, November 8, 2021
Title: Spondyloarthritis Including PsA – Treatment Poster II: Psoriatic Arthritis I (1329–1363)
Session Type: Poster Session C
Session Time: 8:30AM-10:30AM
Background/Purpose: Secukinumab (SEC) has demonstrated long-lasting efficacy and a favorable safety profile in patients (pts) with psoriatic arthritis (PsA) and ankylosing spondylitis (AS) across Phase 3 trials.1, 2 SERENA is an ongoing, longitudinal, observational study in more than 2900 pts with moderate to severe psoriasis, active PsA, and AS conducted at 438 sites across Europe with an expected duration of up to 5 years.3 Here we report data on impact of intermediate treatment interruption on SEC effectiveness in pts with active PsA or AS from the SERENA study.
Methods: This interim analysis included data for 534 PsA and 470 AS pts enrolled in the study between Oct 2016 and Oct 2018 and followed up for at least 2 years. Pts (aged ≥18 years) with active PsA or AS were required to have received at least 16 weeks of SEC treatment before enrolment in the study. A treatment interruption was defined as interruption of SEC therapy for at least 3 months between the last injection and re-initiation. Effectiveness assessments included swollen and tender joint count in PsA pts, and Pt Global Assessment (PtGA) and BASDAI score in AS pts before and during treatment interruption and post SEC re-initiation. Pts with assessments in at least two of the time periods were included. The last assessment prior to the intermediate treatment interruption was used as baseline. The assessment closest to 6 months after re-initiation was considered as post SEC re-initiation assessment.
Results: A total of 31 PsA (5.8%) pts and 42 (8.9%) AS pts had an intermediate treatment interruption since initiation of SEC treatment. The mean (SD) duration of treatment interruption was 24.8 (16.4) and 26.4 (22.9) weeks for PsA and AS pts, respectively. The mean (SD) duration of SEC treatment before the treatment interruption was 86.8 (50.3) and 90.2 (46.9) weeks, and after the treatment interruption was 73.6 (44.4) and 63.2 (46.8) weeks. The most commonly reported reasons included adverse events (AEs) reported in 18 (58.1%) PsA and 19 (45.2%) AS pts, pt decision reported in 3 (9.7%) PsA and 3 (7.1%) AS pts, and COVID-19 outbreak-related reasons reported in 1 (3.2%) PsA and 6 (14.3%) AS pts (Figure 1). More than 80% of PsA pts and 76% of AS pts, reinitiated SEC without a loading phase after the treatment interruption. The swollen and tender joint count increased in PsA pts from the last assessment prior to the treatment interruption [1.3 (1.0) and 7.2 (11.4); n=6] to the first assessment during the treatment interruption [4.0 (1.4) and 16.5 (19.1); n=2], and gradually decreased post SEC re-initiation [0.4 (0.5) and 2.0 (0.7); n=5]. PtGA and BASDAI remained stable in AS pts from the last assessment prior to the treatment interruption to the first assessment during the treatment interruption and after SEC re-initiation (Figure 2).
Conclusion: SEC intermediate treatment interruption occurred due to a variety of reasons in the real-world setting mainly due to AEs and pt decision. The majority of pts re-initiated SEC treatment without a loading phase. No notable impact of the intermediate treatment interruption was observed on the effectiveness of SEC.
References:
1. Mease P, et al. Ann Rheum Dis. 2018;77(6): 890–8972.
2. Pavelka K, et al. Arthritis Res Ther. 2017;19(1):285
3. Kiltz, U et al. Adv Ther 2020; 37:2865–83
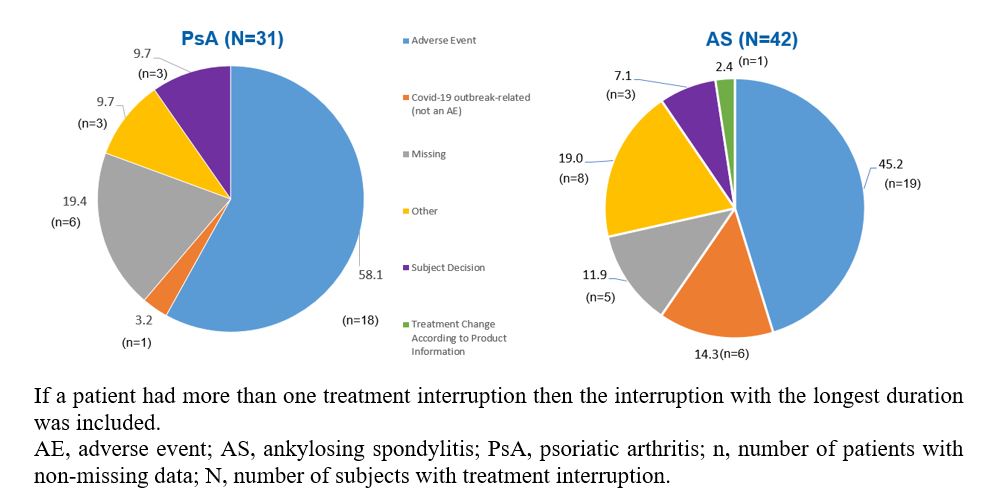 Figure 1: Reasons for a secukinumab treatment interruption in PsA and AS patients
Figure 1: Reasons for a secukinumab treatment interruption in PsA and AS patients
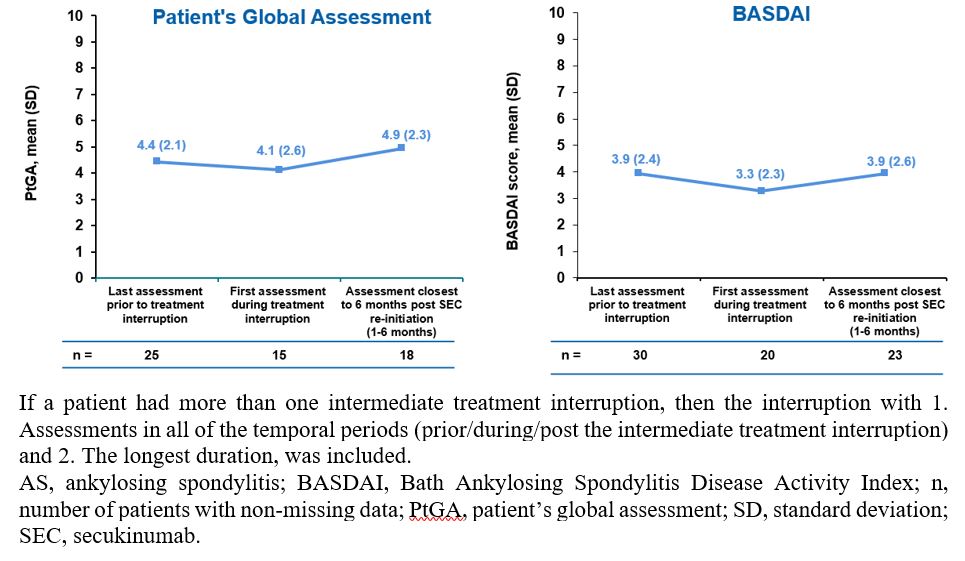 Figure 2: Patient’s Global Assessment and BASDAI score before the treatment interruption, during the treatment interruption and post re-initiation in AS patients
Figure 2: Patient’s Global Assessment and BASDAI score before the treatment interruption, during the treatment interruption and post re-initiation in AS patients
To cite this abstract in AMA style:
Kiltz U, Sfikakis P, Gullick N, Katsifis G, Kandyli A, Brandt-Jrgens J, Goupille P, Maiden N, Gaffney K, Aassi M, Schulz B, Pournara E, Jagiello P. Impact of Intermediate Treatment Interruption on Secukinumab Efficacy in Patients with Active Psoriatic Arthritis and Ankylosing Spondylitis: Interim Analysis Results from the SERENA Study [abstract]. Arthritis Rheumatol. 2021; 73 (suppl 9). https://acrabstracts.org/abstract/impact-of-intermediate-treatment-interruption-on-secukinumab-efficacy-in-patients-with-active-psoriatic-arthritis-and-ankylosing-spondylitis-interim-analysis-results-from-the-serena-study/. Accessed .« Back to ACR Convergence 2021
ACR Meeting Abstracts - https://acrabstracts.org/abstract/impact-of-intermediate-treatment-interruption-on-secukinumab-efficacy-in-patients-with-active-psoriatic-arthritis-and-ankylosing-spondylitis-interim-analysis-results-from-the-serena-study/
