Session Information
Date: Monday, November 8, 2021
Title: SLE – Diagnosis, Manifestations, & Outcomes Poster III: Outcomes (1257–1303)
Session Type: Poster Session C
Session Time: 8:30AM-10:30AM
Background/Purpose: Health-related quality of life (HRQoL) is considered one of the most important outcomes in clinical trials of systemic lupus erythematosus (SLE), along with reduction in disease activity and safety. We studied the duration and consecutiveness of remission or low disease activity throughout a 52-week long period on standard therapy plus belimumab or placebo in relation to HRQoL outcome.
Methods: We analysed pooled 52-week data from the BLISS-52 (N=865) and BLISS-76 (N=819) phase III trials. We determined remission using the prevailing Definitions of Remission in SLE (DORIS) definition (1) and low disease activity using the Lupus Low Disease Activity State (LLDAS) (2). Remission required clinical (c)SLEDAI-2K=0, PhGA (0–3) <0.5, and prednisone ≤5 mg/day. LLDAS required SLEDAI-2K ≤4, PhGA (0–3) ≤1, and prednisone ≤7.5 mg/day. HRQoL was measured with the SF-36 physical and mental component summary (PCS and MCS), and EQ-5D-3L. Minimal clinically important difference (MCID) at week 52 for PCS and MCS was set to 2.5, and for EQ-5D-3L utility index to 0.040. Associations were assessed using quantile regression analysis. Adjustments for demographics, disease duration, organ damage and baseline status were incorporated.
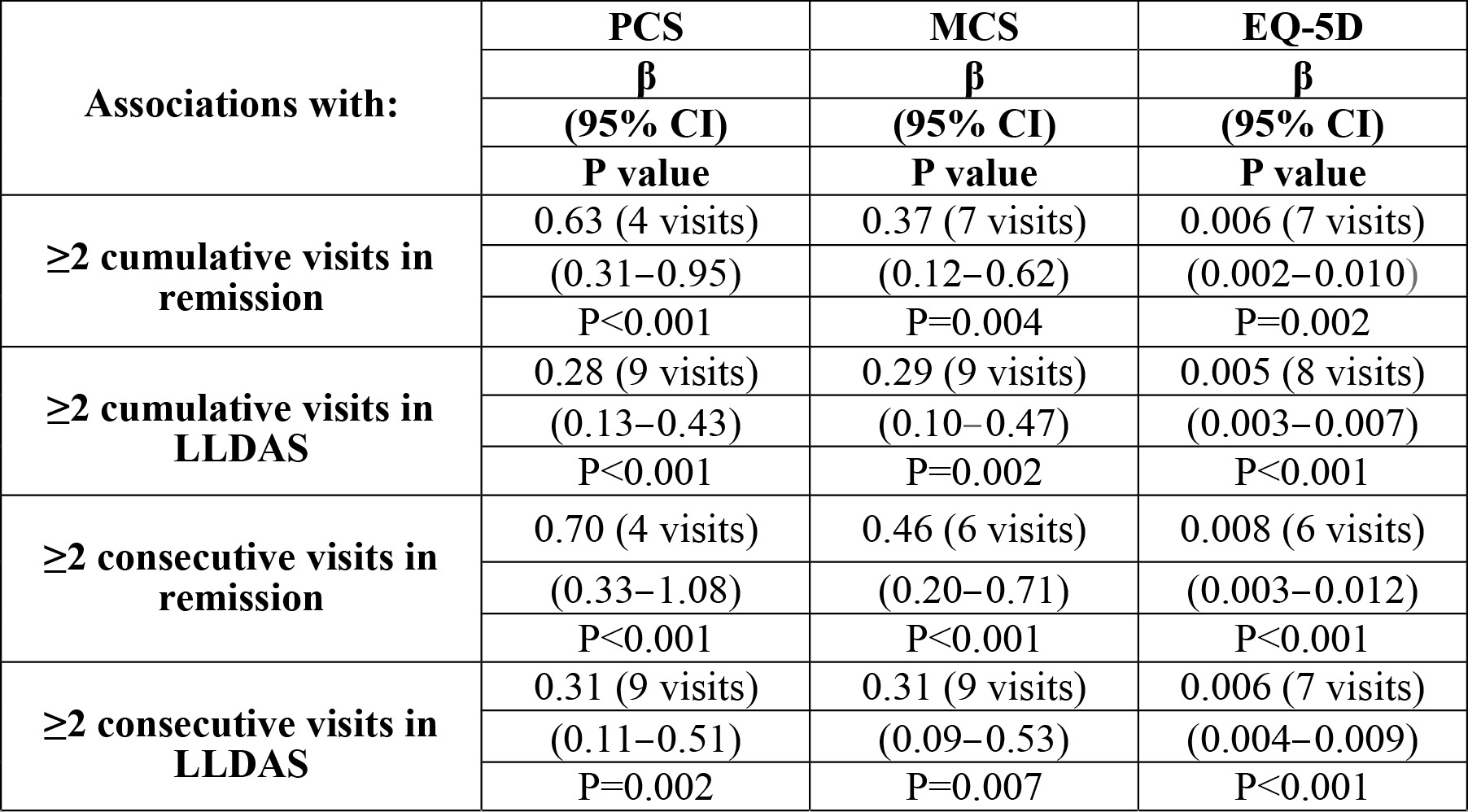
Results: The minimum cumulative attainment of remission to achieve a benefit in PCS ≥MCID at week 52 was four visits (corresponding to 16 weeks) (β=0.63), while 7 visits (28 weeks) were required for MCS differences ≥MCID (β=0.37). Correspondingly, 9 visits in LLDAS (36 weeks) were required for achieving differences ≥MCID in both PCS (b=0.28) and MCS (β=0.29). Table 1 shows 95% confidence intervals and p values. When analysing the impact of sustained remission and LLDAS, four consecutive visits in remission (16 weeks) were required for PCS ≥MCID (b=0.70), whereas six visits (24 weeks) were required for MCS ≥MCID (b=0.46). Sustained LLDAS for nine consecutive visits (36 weeks) was needed for PCS and MCS ≥MCID (b=0.31 and 0.31, respectively). For EQ-5D ≥MCID to be reached, a cumulative total of seven visits (28 weeks) in remission (b=0.006), or eight visits (32 weeks) in LLDAS (b=0.005) was required, whereas if sustained, remission for six visits (24 weeks; b=0.008) or LLDAS for seven visits (28 weeks; b=0.006) were sufficient.
Conclusion: Attainment of remission or LLDAS in the BLISS-52 and BLISS-76 trials of belimumab was associated with improved HRQoL. Less time was required in remission than in LLDAS to achieve clinically important differences in multiple HRQoL aspects. Clinically important differences in HRQoL required shorter total time if the remission or LLDAS was sustained. Clinically important differences in mental aspects of HRQoL required longer time in remission than physical aspects. The impact of cumulative and sustained remission or LLDAS on HRQoL adds evidence on the clinical importance of these treat-to-target endpoints.
References
1) van Vollenhoven R. et al. Ann Rheum Dis. 2017
2) Franklyn K. et al. Ann Rheum Dis. 2016
To cite this abstract in AMA style:
Emamikia S, Oon S, Gomez A, Lindblom J, Borg A, Enman Y, Morand E, Grannas D, van Vollenhoven R, Nikpour M, Parodis I. The Impact of Remission and Low Disease Activity Attainment on Health-related Quality of Life in Two Phase III Clinical Trials of Belimumab in Systemic Lupus Erythematosus [abstract]. Arthritis Rheumatol. 2021; 73 (suppl 9). https://acrabstracts.org/abstract/the-impact-of-remission-and-low-disease-activity-attainment-on-health-related-quality-of-life-in-two-phase-iii-clinical-trials-of-belimumab-in-systemic-lupus-erythematosus/. Accessed .« Back to ACR Convergence 2021
ACR Meeting Abstracts - https://acrabstracts.org/abstract/the-impact-of-remission-and-low-disease-activity-attainment-on-health-related-quality-of-life-in-two-phase-iii-clinical-trials-of-belimumab-in-systemic-lupus-erythematosus/