Session Information
Session Type: Poster Session C
Session Time: 8:30AM-10:30AM
Background/Purpose: This systematic review evaluated all published randomized controlled trials (RCTs) assessing pharmacological and non-pharmacological therapies in patients with hand osteoarthritis (HOA).
Methods: The following electronic data sources were searched from inception to December 2020: MEDLINE, EMBASE, AMED, Clinicaltrials.gov and EBM reviews, including the Cochrane Database of Systematic Reviews (CDSR), Database of Abstracts of Reviews of Effectiveness (DARE), ACP Journal Club, and the Central Cochrane Database. RCTs were included if they evaluated a non-surgical, therapeutic intervention in adult subjects with HOA. The trial must have explicitly stated that a randomized method of allocation to a treatment group was used. RCTs evaluating OA at multiple sites were only included if efficacy data was presented separately for the hand. Exclusion criteria included: RCTs evaluating a surgical therapy, conference proceedings, unpublished RCTs, and non-English RCTs if their English abstracts did not contain sufficient details on trial methodology and outcomes. Study quality was evaluated using the Jadad’s scoring checklist. A meta-analysis would be completed if possible.
Results: 133 RCTs were analyzed in this systematic review. There was no consistent definition of hand OA used in the RCTs, with most trials (N = 102) not explicitly distinguishing between primary (idiopathic) and secondary OA. Sixty-one RCTs used a validated hand OA classification scheme for study entry, with the most common being the ACR classification criteria (N = 54). Radiographs were taken at baseline in seventy-seven RCTs. Most studies described their methods for randomization, blinding and allocation concealment. However, studies underreported features specific to HOA, such as pattern of joint involvement and number of affected joints. Standardized outcome assessments for pain and function were commonly presented, but measures of other HOA specific outcomes, such as health-related quality of life and patient global assessments, were underreported. The mean Jadad score for all entries was 3.08. Increasing Jadad scores were noted over time. A meta-analysis was not completed due to significant heterogeneity amongst high quality RCTs and limited quantity of data.
The following pharmacologic therapies demonstrated efficacy across multiple RCTs: systemic NSAIDs, topical NSAIDs, intramuscular and intravenous clodronate, topical capsaicin, topical trolamine salicylate, oral chondroitin sulfate. Non-pharmacologic therapies that demonstrated efficacy across multiple studies include splints, joint strengthening exercises, mobilization, paraffin baths and multidisciplinary combined intervention. The remainder of the therapies had mixed or negative results, were compared to other therapies in single studies, or efficacy compared to placebo was only demonstrated in a single study.
Conclusion: HOA is a complex area in which to study the efficacy of therapies. Future trials should consistently report on HOA specific features and outcome assessments to make clinically relevant conclusions about the efficacy of the diverse treatment options available.
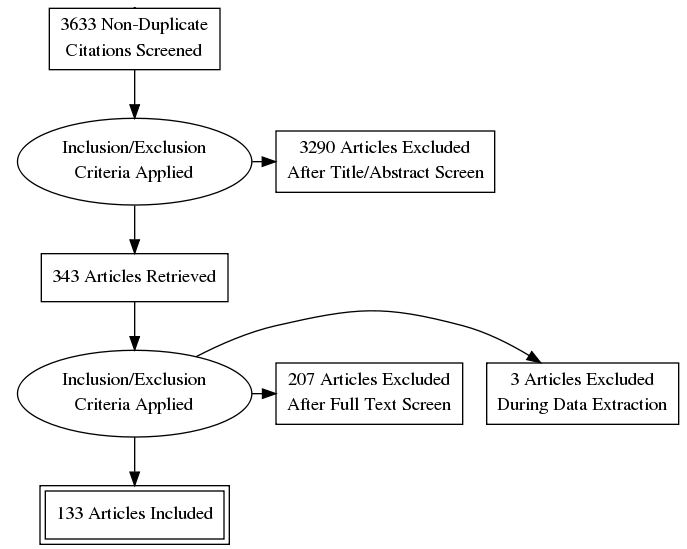 Fig. 1: PRISMA diagram summarizing search strategy, study identification and retrieval.
Fig. 1: PRISMA diagram summarizing search strategy, study identification and retrieval.
To cite this abstract in AMA style:
Mi H, Oh C, Towheed T. Systematic Review of Non-surgical Therapies for Hand Osteoarthritis [abstract]. Arthritis Rheumatol. 2021; 73 (suppl 9). https://acrabstracts.org/abstract/systematic-review-of-non-surgical-therapies-for-hand-osteoarthritis/. Accessed .« Back to ACR Convergence 2021
ACR Meeting Abstracts - https://acrabstracts.org/abstract/systematic-review-of-non-surgical-therapies-for-hand-osteoarthritis/
