Session Information
Date: Sunday, November 7, 2021
Title: Spondyloarthritis Including PsA – Treatment Poster I: Axial Spondyloarthritis (0908–0939)
Session Type: Poster Session B
Session Time: 8:30AM-10:30AM
Background/Purpose: Biological disease-modifying antirheumatic drugs (bDMARDs) should be considered in patients with active axial spondyloarthritis (AxSpA) despite treatment with nonsteroidal anti-inflammatory drugs. The objective of this study was to compare drug retention in patients with active AxSpA treated with tumor necrosis factor inhibitors (TNFi) and secukinumab (SEC), an interleukin 17 inhibitor.
Methods: AxSpA patients from three tertiary care centers starting SEC or TNFi in 2016-2019 were included. Hazard-ratios (HR) for treatment discontinuation were calculated using propensity score and after overlap weighting.
Results: A total of 279 patients were included (TNFi 178, SEC 101). Mean (SD) age was 45.1 (13.1) years and 45.5% were women. Most of them were diagnosed with radiographic sacroiliitis (63.4%), few were bDMARD-naïve (33.7% and 14.9% for TNFi and SEC, respectively). Before matching, patients of the SEC group were older, more likely to present with peripheral joint manifestations, radiographic sacroiliitis, had a higher prevalence of male gender, HLA-B27 positive status, fibromyalgia and comorbidities. 77 matched pairs (mean values of the 10 imputed datasets) were found.
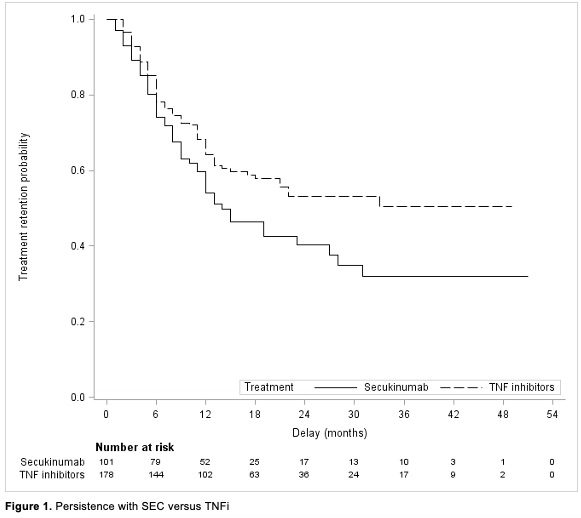
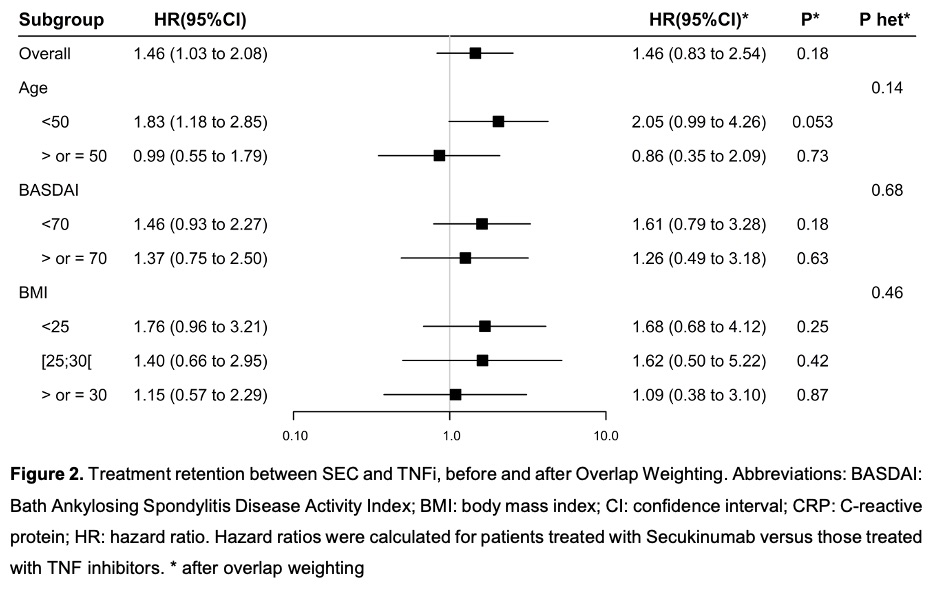
Out of 279 patients, 128 (45.9%) discontinued treatment (Figure 1). In unadjusted analyses, a better drug retention was observed with TNFi compared with SEC (Hazard Ratio HR for treatment discontinuation: 1.46 95% CI: 1.03 to 2.08; p=0.033). The HR for treatment discontinuation in overlap-weighting and matched cohort were 1.46 (95%CI: 0.83 to 2.54; p=0.18) and 1.46 (95%CI: 0.86 to 2.50; p=0.16), respectively. 6-month and 12-month SEC retention rates were 74% and 54%, respectively. Subgroup analyses (age, BASDAI at diagnosis, BMI) showed no difference between TNFi and SEC (Figure 2).
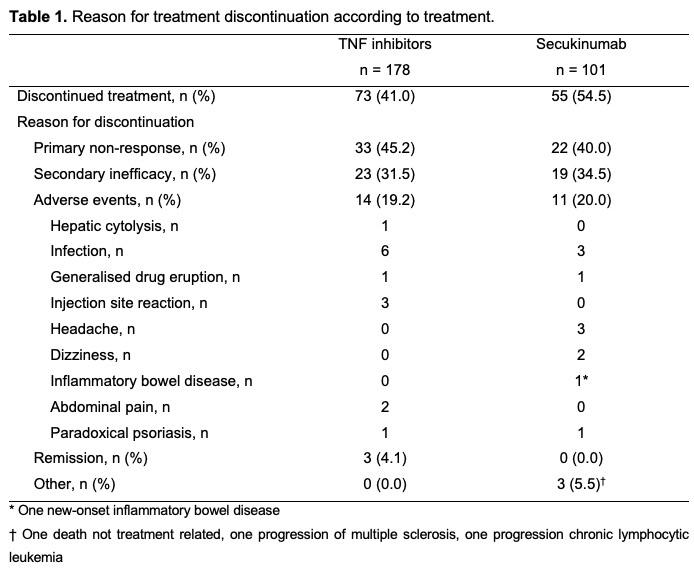
Reasons for discontinuation of TNFi and SEC treatments are demonstrated in Table 1. The main reason for discontinuating both treatments was inefficacy. The overall rate of adverse events leading to treatment discontinuation was similar for both treatment groups (19.2% and 20.0% for TNFi and SEC, respectively).
Conclusion: In this real-world AxSpA cohort, drug retention of SEC and TNFi were similar. In subgroups analyses, age, BASDAI at diagnosis and BMI did not favour any group. These results should be supplemented by head-to-head trials.
To cite this abstract in AMA style:
Delépine T, PHILIPPE P, CAILLIAU E, HOUVENAGEL E, Pascart T, DEPREZ X, FLIPO R, LETAROUILLY J. Drug Retention of Tumor Necrosis Factors and IL-17 Inhibitors in Axial Spondyloarthritis: A Multi-Center Comparative Analysis [abstract]. Arthritis Rheumatol. 2021; 73 (suppl 9). https://acrabstracts.org/abstract/drug-retention-of-tumor-necrosis-factors-and-il-17-inhibitors-in-axial-spondyloarthritis-a-multi-center-comparative-analysis/. Accessed .« Back to ACR Convergence 2021
ACR Meeting Abstracts - https://acrabstracts.org/abstract/drug-retention-of-tumor-necrosis-factors-and-il-17-inhibitors-in-axial-spondyloarthritis-a-multi-center-comparative-analysis/