Session Information
Date: Sunday, November 7, 2021
Title: SLE – Diagnosis, Manifestations, & Outcomes Poster II: Manifestations (0855–0896)
Session Type: Poster Session B
Session Time: 8:30AM-10:30AM
Background/Purpose: Preliminary data in people with SLE suggested that disease activity as well as SLE treatment at time of COVID-19 acquisition impact COVID-19 outcomes over and above other known risk factors. We assessed characteristics associated with poor outcomes in a global population of people with SLE and COVID-19.
Methods: People with SLE reported in the COVID-19 Global Rheumatology Alliance (GRA) physician-reported registry from March 24th 2020 to April 12th 2021 were included. Variables collected included age, gender, region [Europe, North America, South America and other (Africa, Asia and Australia)], comorbidities (chronic renal disease, cardiovascular disease, and the number of other comorbidities), physician global assessment of disease activity, calendar period, glucocorticoid dose, and SLE treatment at the time of COVID diagnosis. SLE treatment was categorized into five groups: antimalarials only (reference), no SLE drugs, non-biologic immunosuppressant (IS) monotherapy, biologics/target synthetic IS, and combination IS therapy. An ordinal outcome was defined as: 1) not hospitalized, 2) hospitalized without supplementary oxygen or with non-invasive ventilation, 3) hospitalized with mechanical ventilation/extracorporeal membrane oxygenation and 4) death. We constructed a multivariable ordinal logistic regression model to assess the relationship between COVID-19 severity and demographic characteristics, comorbidities, medications and disease activity.
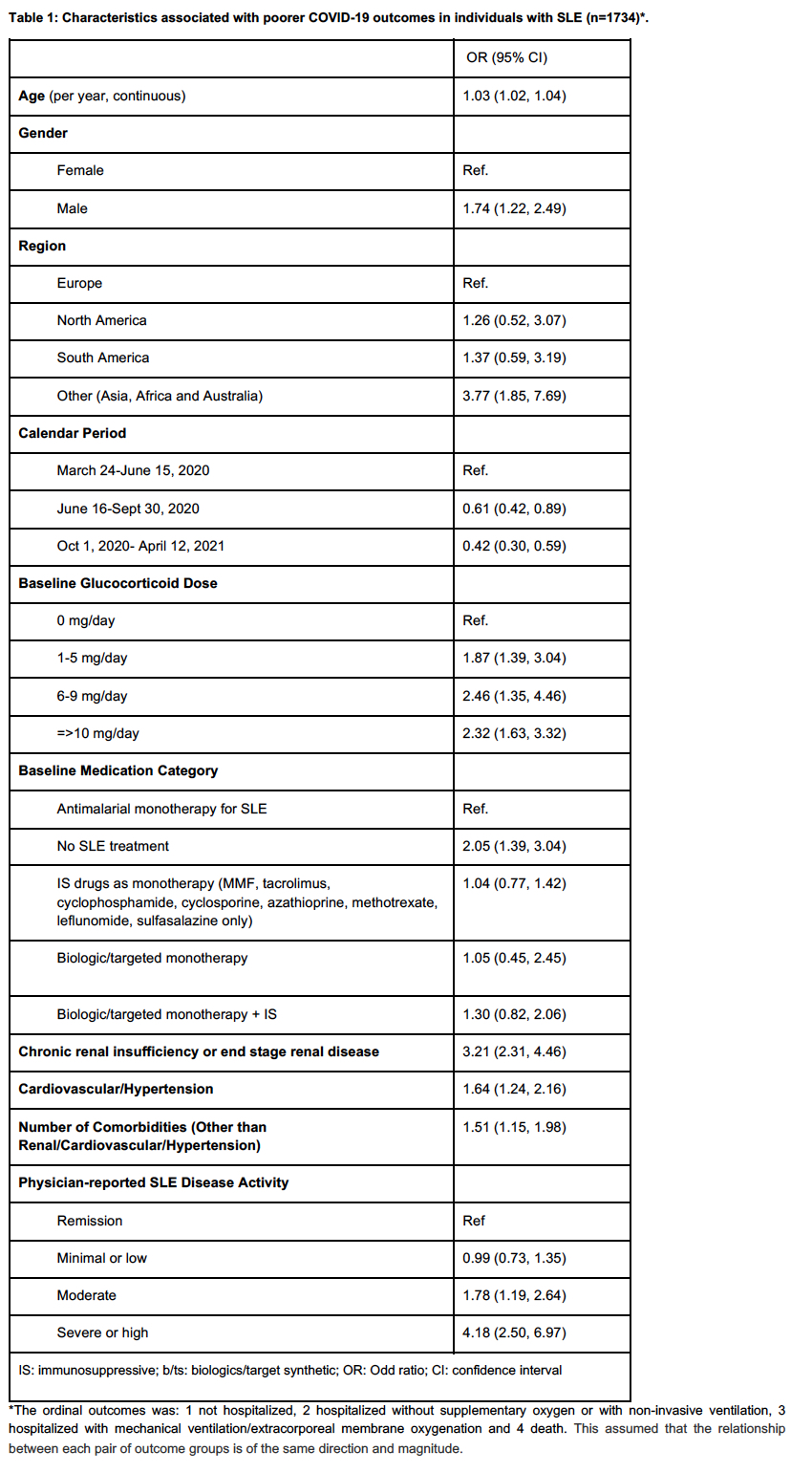
Results: 1734 patients were included; 1567 (90.4%) were female, median age was 44.3 (SD: 14.3) years. A total of 1291 (74.5%) patients were not hospitalized; 148 (8.5%) patients were hospitalized without oxygen or with non-invasive ventilation, 195 (11.2%) patients were hospitalized with mechanical ventilation/extracorporeal membrane oxygenation and 100 (5.6%) died. In our multivariable model, more severe COVID-19 outcomes were seen in older patients (odds ratio, OR=1.03 per year), males (OR =1.74), patients outside Europe and North and South America (OR=3.77), patients on prednisone (0-5 mg/d OR=1.87, 5-10 mg/d OR=2.46, and >10 mg/d OR=2.32), no SLE therapy (OR =2.05), chronic renal disease (OR =3.21), cardiovascular disease (OR =1.64), the number of other comorbidities (OR=1.51) and moderate and high disease activity (OR =1.78 and OR=4.18, respectively). There was evidence of a calendar period effect, with worse outcomes in those with a COVID diagnosis earlier in the pandemic (before June 15, 2020); these data are summarized in Table 1.
Conclusion: These results demonstrate patterns similar to the general rheumatic disease population, and underscore the importance of controlling disease activity in people
with SLE patients during the COVID-19 pandemic.
To cite this abstract in AMA style:
Ugarte-Gil M, Alarcn G, Seet A, Izadi Z, Duarte-Garcia A, Reategui-Sokolova C, Clarke A, Wise L, Pons-Estel G, José Santos M, Bernatsky S, Ribeiro S, Al Emadi S, Sparks J, Hsu T, D'Silva K, Patel N, Gilbert E, Valenzuela-Almada M, Jnsen A, Landolfi G, Fredi M, Goulenok T, Devaux M, Mariette X, Queyrel V, Romão V, Sequeira G, Hasseli R, Hoyer B, Voll R, Specker C, Baez R, Castro Coello V, Neto E, Ferreira G, Andre Monticielo O, Sirotich E, Liew J, Hausmann J, Sufka P, Grainger R, Bhana S, Costello W, Wallace Z, Jacobsohn L, Strangfeld A, Frazão Mateus E, Hyrich K, Gossec L, Carmona L, Lawson-Tovey S, Kearsley-Fleet L, Schaefer M, Machado P, Robinson P, Gianfrancesco M, Yazdany J. Characteristics Associated with Poor COVID-19 Outcomes in People with Systemic Lupus Erythematosus (SLE): Data from the COVID-19 Global Rheumatology Alliance (GRA) [abstract]. Arthritis Rheumatol. 2021; 73 (suppl 9). https://acrabstracts.org/abstract/characteristics-associated-with-poor-covid-19-outcomes-in-people-with-systemic-lupus-erythematosus-sle-data-from-the-covid-19-global-rheumatology-alliance-gra/. Accessed .« Back to ACR Convergence 2021
ACR Meeting Abstracts - https://acrabstracts.org/abstract/characteristics-associated-with-poor-covid-19-outcomes-in-people-with-systemic-lupus-erythematosus-sle-data-from-the-covid-19-global-rheumatology-alliance-gra/