Session Information
Date: Sunday, November 7, 2021
Session Type: Poster Session B
Session Time: 8:30AM-10:30AM
Background/Purpose: Short-term glucocorticoid (GC) bridging therapy results in rapid suppression of disease activity during the initial treatment of rheumatoid arthritis (RA) patients with DMARDs. But there are concerns that many patients may not be able to discontinue GC, risking adverse events. We reviewed the literature to investigate the success rate of GC discontinuation during 24 months of follow-up in trials using GC as bridging therapy.
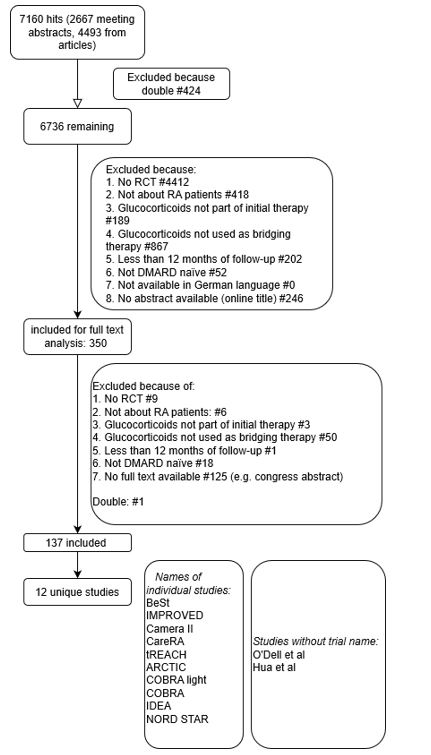
Methods: A systematic literature search was conducted in PubMed, Embase, Web of Science, COCHRANE library, Emcare and Academic Search Premier to find clinical trials about RA patients treated with (also) initial GC bridging with at least 12 months follow-up. Studies were excluded according to a decision rule (see flowchart in figure 1). Heterogeneity was assessed based on pre-defined items, describing patient characteristics, initial GC bridging scheme, additional treatment and treatment adjustment protocols. Studies were assessed for continuation or restarting of GC after the induction scheme.
Results: The literature search identified 7160 abstracts (flowchart in figure 1). Based on reviewing the first 100 abstracts, we found a 97% interobserver agreement between the 3 reviewers (LO, ISN and SAB). The remaining abstracts were screened separately by the reviewers. 350 abstracts were included for full text analysis, resulting in 12 unique studies (table 1). The majority of included patients fulfilled the ACR/EULAR 2010 (53%) or the ACR 1987 (33%) criteria. At baseline, the mean symptom duration ranged from 4 to 7 months and the disease activity score (DAS) ranged from 3.0 to 5.2. All trials started with a DMARD at baseline next to the GC. In 83% of the studies oral GC bridging was used (initial dose ranging between 7.5 and 60 mg/day, the latter always followed by rapid tapering to 7.5 mg/day as maintenance dose, see table 1). Studies starting with a lower initial dose (5 studies) tapering to 5 mg/day (2/5, 40%) or directly to zero (3/5, 60%). Only 4/12 studies reported data on GC use after the GC induction phase, either at 12 (3/4) and/or at 24 (3/4) months follow-up (see table 2). The proportion of patients still using GC ranged from 23 to 44% at 12 months and 10 to 28% at 24 months. One study only started tapering at 12 months; at 24 months 45% of the patients were still using GCs (see table 2). Other outcome measures (e.g. cumulative or average GC dose, number of GC episodes) were reported in even fewer studies. Therefore, a meta-analysis was not performed.
Conclusion: The currently available data do not provide sufficient information on successful GC discontinuation after the induction phase, since initial type and dose of GC tapering schedules differed between studies and the total number of trials with pertinent data was small. However, preliminary data suggest that in the clinical trials assessed, up to 60% of patients have discontinued GCs initially at 12 months follow-up, and up to 70% at 24 months.
To cite this abstract in AMA style:
van Ouwerkerk L, Nevins I, Verschueren P, Smolen J, Landewé R, Bijlsma J, Kerschbaumer A, Huizinga T, Westhovens R, Allaart C, Bergstra S. What Is the Success Rate in Clinical Trials of Discontinuation Glucocorticoids After Their Use as Bridging Therapy – a Systematic Literature Review [abstract]. Arthritis Rheumatol. 2021; 73 (suppl 9). https://acrabstracts.org/abstract/what-is-the-success-rate-in-clinical-trials-of-discontinuation-glucocorticoids-after-their-use-as-bridging-therapy-a-systematic-literature-review/. Accessed .« Back to ACR Convergence 2021
ACR Meeting Abstracts - https://acrabstracts.org/abstract/what-is-the-success-rate-in-clinical-trials-of-discontinuation-glucocorticoids-after-their-use-as-bridging-therapy-a-systematic-literature-review/