Session Information
Date: Sunday, November 7, 2021
Session Type: Poster Session B
Session Time: 8:30AM-10:30AM
Background/Purpose: Identifying factors associated with treatment response and remission in patients with RA may assist with therapeutic decision-making. An international observational study evaluated efficacy and safety in patients with RA who initiated intravenous (IV) abatacept (AbataCepT In rOutiNe clinical practice [ACTION]; NCT02109666) and noted retention of 47.9% at 2 years.1 This post hoc analysis identified baseline characteristics predictive of remission following abatacept treatment in patients with RA who participated in the real-world setting ACTION study.
Methods: Patients (aged ≥ 18 years) with moderate-to-severe RA from ACTION who initiated IV abatacept as first- or ≥ second-line therapy and achieved DAS28 (CRP; < 2.6), Clinical Disease Activity Index (CDAI; ≤ 2.8) or Simplified Disease Activity Index (SDAI; ≤ 3.3) remission at 12 months were included. Univariate logistic regression analysis was performed to determine potential predictors of remission by treatment arm including baseline demographics, disease characteristics, serum markers, and previous treatments. Significant variables (P < 0.2) from the univariate analyses were evaluated by a multivariate backward elimination logistic regression model; odds ratios (95% confidence intervals) and P values were determined. Standardized values were used for the continuous baseline predictors.
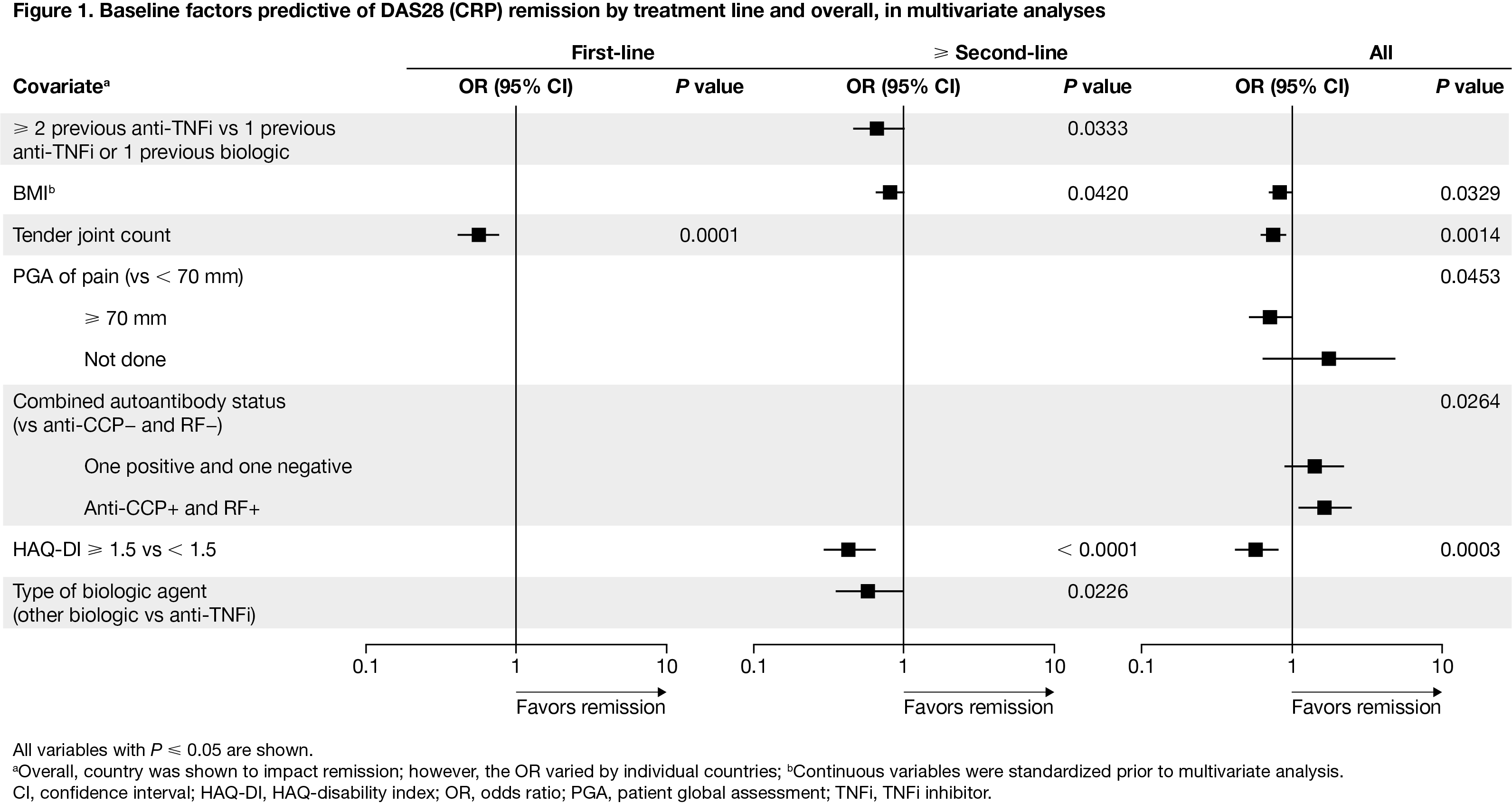
Results: Overall, 2260/2364 enrolled patients were evaluable (643 first-line and 1617 ≥ second-line abatacept). For the overall population, DAS28 (CRP) (Figure 1) and CDAI remission (Figure 2) were more likely to be achieved in patients with seropositivity, while DAS28 (CRP) and SDAI remission (Figure 3) were more likely to be achieved in patients with less severe disability (HAQ-disability index [DI]). DAS28 (CRP) remission was more likely to be achieved with ≥ second-line therapy in patients who had low BMI, less severe HAQ-DI, and prior TNF inhibitor (TNFi) use (vs other biologic use). Lastly, in patients who received abatacept as ≥ second-line therapy, CDAI and SDAI remission were more likely to be achieved by patients with: seropositivity, less severe HAQ-DI, shorter RA duration, prior TNFi use (vs other biologic use), and lack of efficacy on last biologic.
Conclusion: This post hoc, multivariate analysis of the ACTION study showed several baseline factors, including seropositive RA and less severe disability (HAQ-DI), to be associated with remission at 12 months for patients treated with IV abatacept. These data support the earlier use of abatacept in patients with seropositive RA.
Reference: 1. Alten R, et al. Clin Rheumatol 2019;38:1413–1424.
Medical writing: Fiona Boswell, PhD (Caudex), funded by Bristol Myers Squibb
To cite this abstract in AMA style:
Alten R, Mariette X, Galeazzi M, Chartier M, Rauch C, Elbez Y, Lozenski K. Baseline Characteristics Predictive of Remission in Patients with RA Following Treatment with IV Abatacept: Post Hoc Analysis of a Real-world Observational Study [abstract]. Arthritis Rheumatol. 2021; 73 (suppl 9). https://acrabstracts.org/abstract/baseline-characteristics-predictive-of-remission-in-patients-with-ra-following-treatment-with-iv-abatacept-post-hoc-analysis-of-a-real-world-observational-study/. Accessed .« Back to ACR Convergence 2021
ACR Meeting Abstracts - https://acrabstracts.org/abstract/baseline-characteristics-predictive-of-remission-in-patients-with-ra-following-treatment-with-iv-abatacept-post-hoc-analysis-of-a-real-world-observational-study/