Session Information
Session Type: Poster Session B
Session Time: 8:30AM-10:30AM
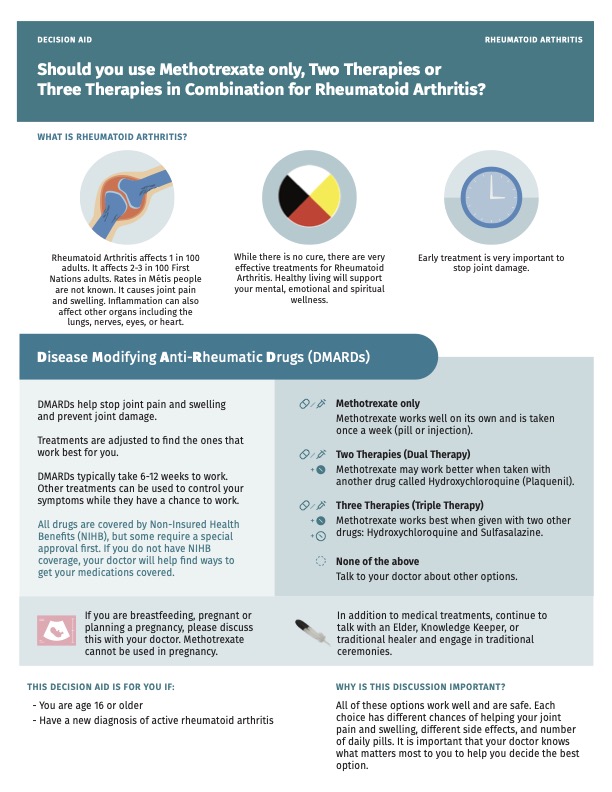
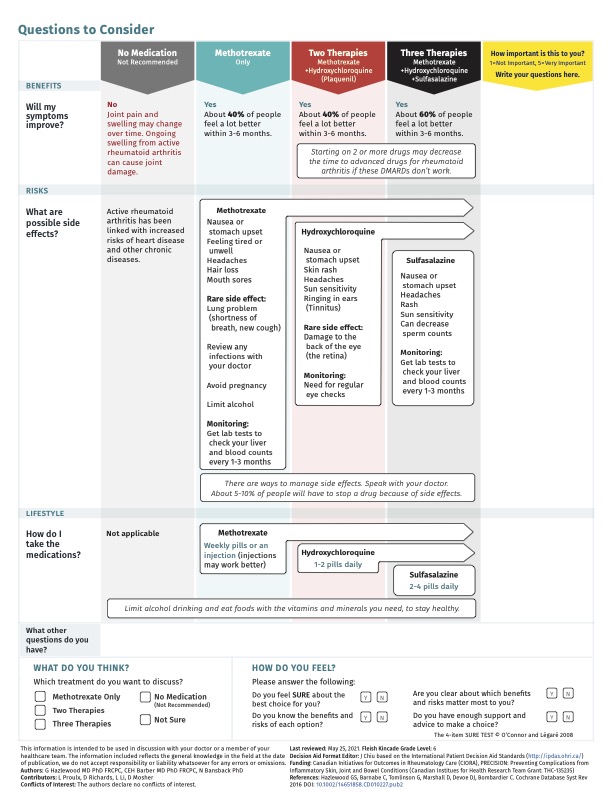
Background/Purpose: Patient decision aids (PtDA) can enable shared decision-making between patients and healthcare providers. We have previously developed a PtDA for first-line methotrexate-based treatment options in rheumatoid arthritis (RA). Adaptations to PtDAs for use with populations facing inequities in healthcare can improve the relevancy of information presented, incorporate appropriate cultural context, and address health literacy concerns. Our objective was to adapt the Early RA PtDA for use with Canadian Indigenous patients making initial disease-modifying treatment choices.
Methods: The Early RA PtDA was modified through an iterative process using data obtained from semi-structured interviews with Indigenous patients with RA using an interview guide. A thematic analysis of interview transcriptions was completed using NVivo 12 software. Initial modifications were made based on the input of a first cohort of patients, with the revised Early RA PtDA verified by a second cohort of patients.
Results: The initial directions for modification were provided by 7 participants, while 9 participants were recruited for the verification cohort. All participants were women living with RA and self-identified as First Nations, Plains Cree, or Indigenous. In the first iteration, the revisions made were to 1) clarify medication names and routes of administration; 2) include Indigenous traditional healing practice options; 3) provide information on formulary coverage for Indigenous patients; 4) simplify text; and 5) include Indigenous images, and use colors aligned with Canadian Indigenous community representation. The second cohort perceived the revised Early RA PtDA to be acceptable for layout and information content, with appreciation expressed by participants for including Indigenous traditional healing practices as adjuncts to treatment, which was seen as beneficial for advancing communication and relationship building with health practitioners. Additional revisions were requested to increase text legibility, insert more Indigenous images, make further changes in colors, address formulary coverage for non-status First Nations patients, and include information on lifestyle factors in managing RA. Indigenous patient-specific evidence on the efficacy and safety of medication options should be included in future iterations if data available, and translation of key words into the end-users’ Indigenous languages should be included for implementation of the PtDA.
Conclusion: Incorporating Indigenous-specific adaptations in the design of PtDAs may increase use and relevancy to support patient focused care and engagement in treatment decisions, thereby supporting health-equity oriented health service interventions.
To cite this abstract in AMA style:
Umaefulam V, Fox T, Hazlewood G, Bansback N, Barber C, Barnabe C. Adaptation of a Shared Decision-Making Tool for Early Rheumatoid Arthritis Treatment Decisions with Indigenous Patients [abstract]. Arthritis Rheumatol. 2021; 73 (suppl 9). https://acrabstracts.org/abstract/adaptation-of-a-shared-decision-making-tool-for-early-rheumatoid-arthritis-treatment-decisions-with-indigenous-patients/. Accessed .« Back to ACR Convergence 2021
ACR Meeting Abstracts - https://acrabstracts.org/abstract/adaptation-of-a-shared-decision-making-tool-for-early-rheumatoid-arthritis-treatment-decisions-with-indigenous-patients/