Session Information
Date: Saturday, November 6, 2021
Title: Miscellaneous Rheumatic & Inflammatory Diseases Poster I (0183–0209)
Session Type: Poster Session A
Session Time: 8:30AM-10:30AM
Background/Purpose: Autoinflammatory periodic fever syndromes (PFS) are characterized by severe systemic and organ inflammation. In clinical trials, successful treatment was achieved with the interleukin-1β inhibitor canakinumab (CAN).
The present study explores the long-term efficacy and safety of CAN in routine clinical practice conditions in pediatric (age ≥2 years) and adult patients with CAPS (cryopyrin-associated periodic syndromes), FMF (familial Mediterranean fever), TRAPS (tumor necrosis factor receptor-associated periodic syndrome) and HIDS/MKD (hyperimmunoglobulinemia D syndrome/mevalonate kinase deficiency).
Methods: RELIANCE is a prospective, non-interventional, observational study based in Germany. Patients with clinically confirmed diagnoses of PFS routinely receiving CAN are enrolled. Besides efficacy parameters regarding disease activity and remission, safety parameters were recorded at baseline and assessed at 6-monthly intervals.
Results: Here we present the interim analysis of 168 patients with PFS enrolled in the RELIANCE Registry between October 2017 and December 2020. Mean age in this cohort was 24.7 years (2-79 years) and the proportion of female patients was 51 %. At baseline, median duration of prior CAN treatment was 3 years (0-12 years).
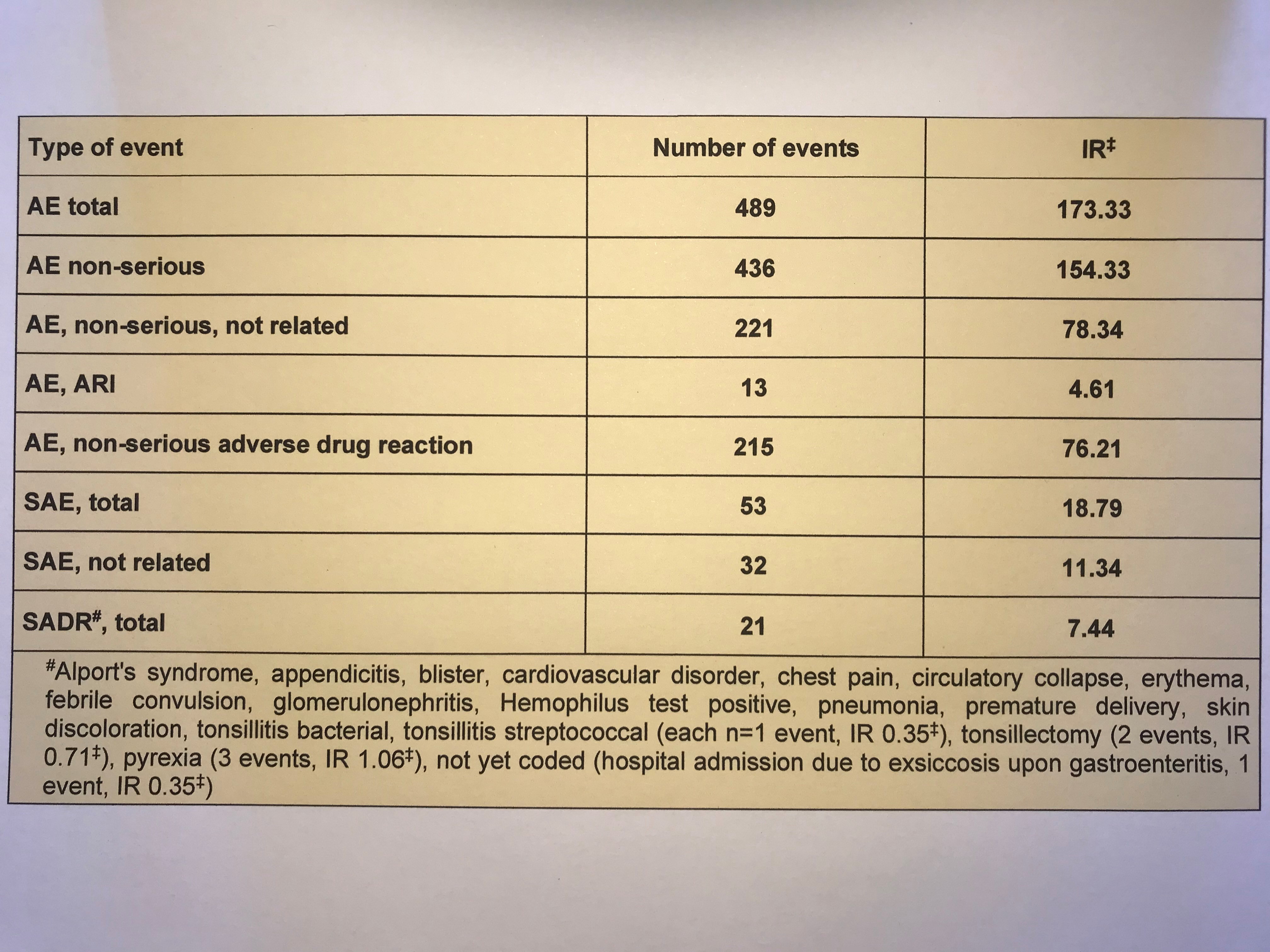
A total of 101 patients (60%) experienced any adverse event (AE) and 22 patients (13%) were affected by severe adverse events (SAE). In 9 patients (5%) SAE were classified as drug related. Of 489 AE, 53 were severe and a total of 21 SAE were classified as treatment-related (table). Overall, 13 AE comprised upper respiratory tract infections (ARI).
Conclusion: The interim data from the RELIANCE study, the longest running real-life canakinumab registry for autoinflammatory periodic fever syndromes, confirm safety of long-term canakinumab treatment across the entire study population.
‡IR, incidence rate per 100 patient years; AE, adverse event; SAE, severe adverse event, SADR, severe adverse drug reaction
To cite this abstract in AMA style:
Kuemmerle-Deschner J, Henes J, Kortus-Goetze B, Kallinich T, Oommen P, Rech J, Weller-Heinemann F, Horneff G, Foeldvari I, Janda A, Schuetz C, Dressler F, Borte M, Hufnagel M, Braner A, Meier F, Fiene M, Weber-Arden J, Blank N. Long-term Safety of Canakinumab in Patients with Autoinflammatory Periodic Fever Syndromes – Interim Analysis of the RELIANCE Registry [abstract]. Arthritis Rheumatol. 2021; 73 (suppl 9). https://acrabstracts.org/abstract/long-term-safety-of-canakinumab-in-patients-with-autoinflammatory-periodic-fever-syndromes-interim-analysis-of-the-reliance-registry/. Accessed .« Back to ACR Convergence 2021
ACR Meeting Abstracts - https://acrabstracts.org/abstract/long-term-safety-of-canakinumab-in-patients-with-autoinflammatory-periodic-fever-syndromes-interim-analysis-of-the-reliance-registry/