Session Information
Date: Saturday, November 6, 2021
Title: Epidemiology & Public Health Poster I: COVID-19 & Vaccination (0084–0117)
Session Type: Poster Session A
Session Time: 8:30AM-10:30AM
Background/Purpose: At the outset of the SARS-CoV-2 pandemic, it was speculated that SLE patients may be at significant risk of developing COVID-19 due to underlying immune dysregulation and use of immunomodulatory therapies. Our aims were to:
1) Compare SARS-CoV-2 seroprevalence pre- and intra-pandemic in a local SLE cohort to (a) ambulatory individuals tested for autoantibodies, and (b) a geographically similar general population.
2) Assess rates of RT-PCR testing and positivity.
3) Evaluate relationships between SARS-CoV-2 exposure and demographic and clinical features in the SLE cohort.
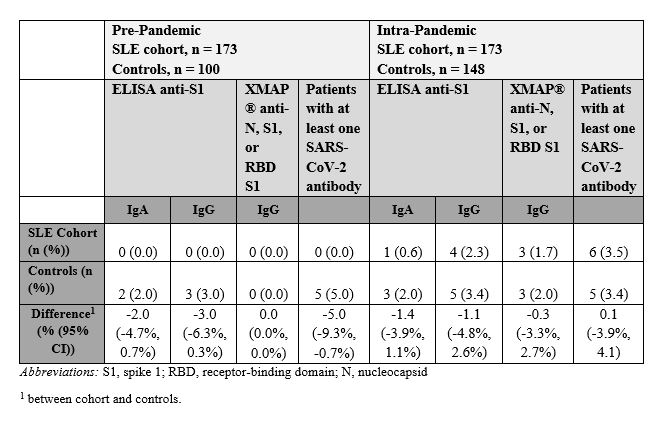
Methods: Sera from 173 SLE patients were included, with pre-pandemic samples biobanked prior to 01/01/2020 and intra-pandemic samples collected from 03/15/2020 to 01/31/2021. One hundred pre-pandemic and 148 intra-pandemic sequential, unselected sera from ambulatory individuals served as controls. All sera were tested for SARS-CoV-2 antibodies using two FDA early use authorized assays: an ELISA measuring IgA and IgG anti-spike 1 (S1) (Euroimmun AG, Lübeck, Germany) and an xMAP® assay detecting IgG antibodies to nucleocapsid (N), S1, and receptor-binding domain (RBD) of S1 (Luminex Corp., Austin, TX). The seroprevalence rate of a geographically similar general population was obtained from online published jurisdiction rates. RT-PCR testing was performed in the SLE cohort by a government-sanctioned laboratory. Differences in seropositivity between cohort and controls, and differences in demographic and clinical features between SLE patients with and without SARS-CoV-2 exposure (defined as positive RT-PCR and/or antibodies to SARS-CoV-2) were assessed using 95% confidence intervals (CI).
Results: The SLE cohort was 95% female with a mean age of 48.5 years (SD 14.7) and mean disease duration of 11.7 years (SD 11.3) (Table 1). Eighty-six percent were using anti-malarials, 29% corticosteroids, and 45% other immunomodulators.
In the pre-pandemic samples, none of the SLE cohort vs. 5 (5%) of the ambulatory controls had at least one antibody to any SARS-CoV-2 antigens (difference (dif) -5.0%, 95% CI -9.3, -0.7%; Table 2). In the intra-pandemic samples, 6 (3.5%) SLE patients vs. 5 (3.4%) of the ambulatory controls (dif 0.1%, 95% CI -3.9%, 4.1%) and 2.9% of the geographically similar general population (dif 0.6%, 95% CI -2.2%, 3.4%) had at least one SARS-CoV-2 antibody. Eighty-one (47%) SLE patients received an RT-PCR test, of which 6/81 (7%) were positive.
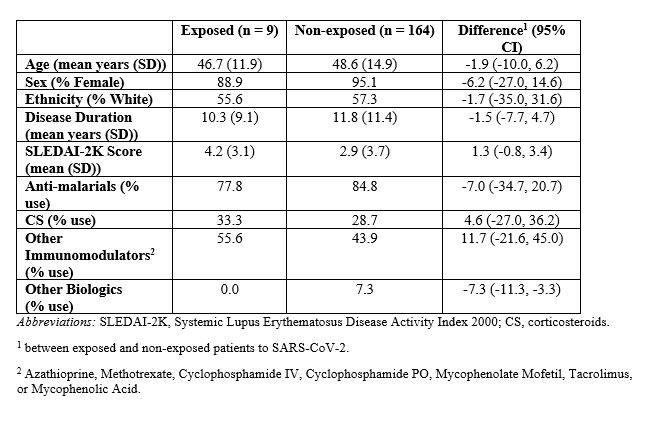
Within the SLE cohort, the only significant difference in pre-pandemic characteristics between those with and without SARS-CoV-2 exposure was biologic use (0.0% vs. 7.3%; dif -7.3, 95% CI -11.3, -3.3) (Table 3).
Conclusion: Despite underlying immune dysregulation and frequent use of immunomodulatory therapies, our SLE patients did not appear to have higher exposure to SARS-CoV-2 or greater risk of developing COVID-19 compared to the general population. Further, none of the SLE patients had pre-pandemic (i.e., cross-reactive) antibodies to SARS-CoV-2 N, S1, or RBD proteins. More effective use of infection control principles may contribute to reducing SARS-CoV-2 infections in SLE.
To cite this abstract in AMA style:
Mathew H, Choi M, Buhler K, Clarke A, Gukova X, Cardwell F, Fritzler M. SARS-CoV-2 Seroprevalence and Seroconversion in a Systemic Lupus Erythematosus Cohort and Comparison to General Population Controls [abstract]. Arthritis Rheumatol. 2021; 73 (suppl 9). https://acrabstracts.org/abstract/sars-cov-2-seroprevalence-and-seroconversion-in-a-systemic-lupus-erythematosus-cohort-and-comparison-to-general-population-controls/. Accessed .« Back to ACR Convergence 2021
ACR Meeting Abstracts - https://acrabstracts.org/abstract/sars-cov-2-seroprevalence-and-seroconversion-in-a-systemic-lupus-erythematosus-cohort-and-comparison-to-general-population-controls/