Session Information
Date: Monday, November 9, 2020
Title: Spondyloarthritis Including Psoriatic Arthritis – Treatment II: Emerging Therapies (2023–2027)
Session Type: Abstract Session
Session Time: 11:00AM-11:50AM
Background/Purpose: Upadacitinib (UPA), a Janus kinase (JAK) inhibitor, was efficacious and well tolerated vs placebo (PBO) during the first 14 weeks (wks) of the phase 2/3 SELECT-AXIS 1 study in patients (pts) with active ankylosing spondylitis (AS) who had an inadequate response to NSAIDs.1 The objective of this interim analysis was to report efficacy and safety of UPA through 1 year in the SELECT-AXIS 1 study.
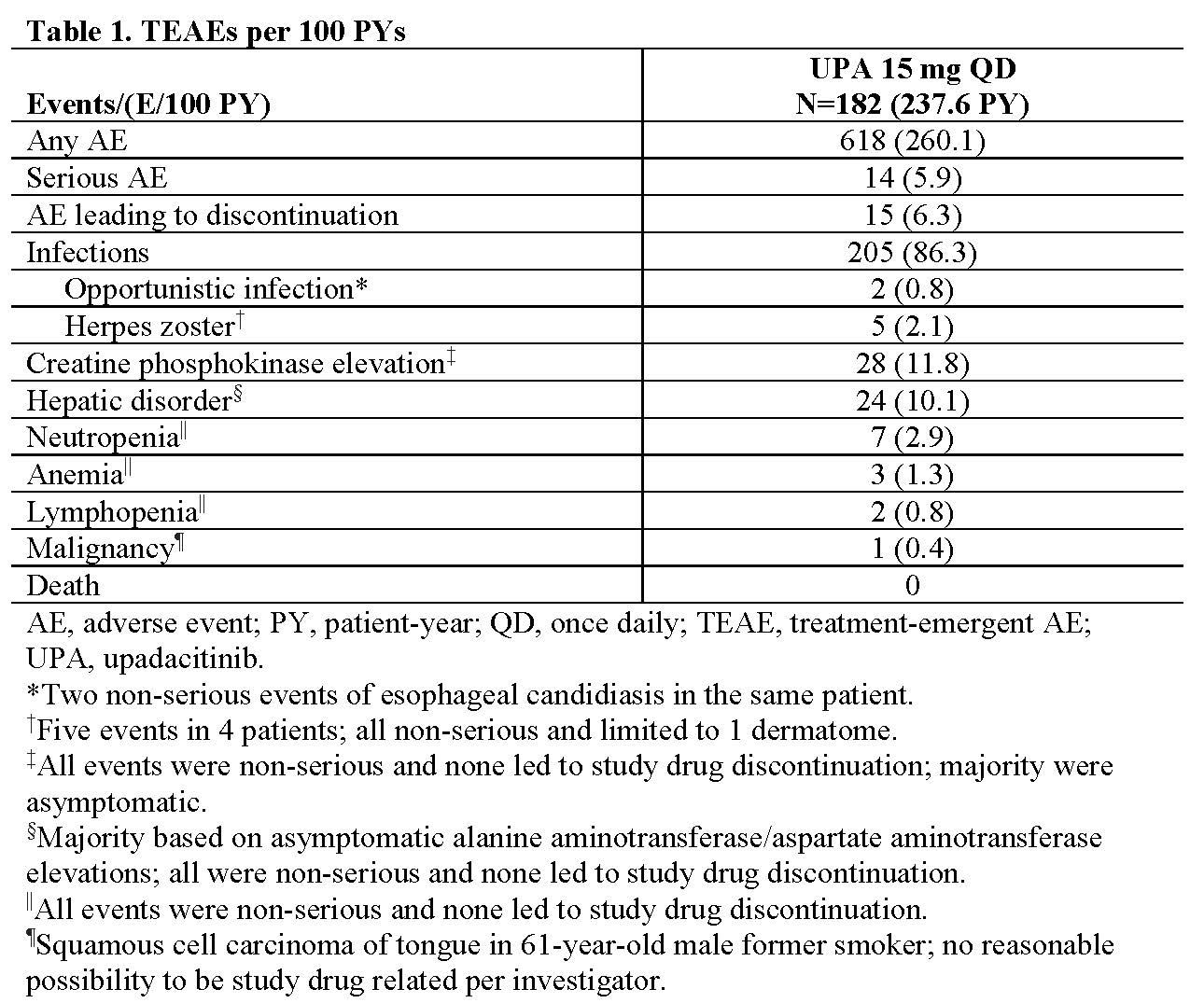
Methods: SELECT-AXIS 1 (NCT03178487) included a randomized, placebo-controlled, 14-wk period followed by 90-wk open-label extension; reported here are data up to wk 64. The study enrolled pts (≥18 y) with active AS (defined as BASDAI ≥4 and pt assessment of back pain ≥4 [numeric rating scale, 0–10] at screening and baseline [BL]) who had an inadequate response to ≥2 NSAIDs or intolerance to or contraindication for NSAIDs and were biologic DMARD naive. At BL, pts were randomized 1:1 to UPA 15 mg once daily (QD) or PBO; at wk 14, pts continued in the open-label extension and received UPA 15 mg QD. Efficacy assessments included the percentage of pts with Assessment of SpondyloArthritis international Society (ASAS) 20/40 response, ASAS partial remission, BASDAI50, and AS Disease Activity Score (ASDAS) responses over time and as change from BL in ASDAS and BASFI. Data are reported both as observed and by using non-responder imputation (NRI) for binary or mixed-effect model repeated measures for continuous efficacy endpoints for missing data. Treatment-emergent adverse events (TEAEs) were monitored throughout the study and reported as events per 100 patient-years (PY) up to January 31, 2020.
Results: Of 187 pts, 178 pts (each n=89 for UPA and PBO arms) completed wk 14 on study drug and entered the open-label extension; 160 pts completed wk 64. Efficacy was maintained or continued to improve throughout the study in the continuous UPA group: 85% (95% CI, 77%–93%) of pts achieved ASAS40 at week 64 in the as-observed analysis and 72% (63%–81%) in the NRI analysis (Figure). Pts who switched from PBO to UPA at wk 14 showed a similar speed of onset and magnitude of response compared with pts who were initially randomized to UPA: 81% (95% CI, 72%–89%) of pts in the as-observed analysis and 70% (61%–80%) of pts in the NRI analysis achieved ASAS40 at wk 64 (Figure). Similar results were observed for other efficacy endpoints (Figure). Among all 182 pts receiving UPA (237.6 PY), 618 AEs (260.1/100 PY) were reported. AEs leading to discontinuation (15 events [6.3/100 PY]) and serious AEs (14 events [5.9/100 PY]) were low (Table 1). No serious infections, active tuberculosis, venous thromboembolic events, gastrointestinal perforation, major adverse cardiovascular events, renal dysfunction, or deaths were reported.
Conclusion: UPA 15 mg QD showed sustained and consistent efficacy over 1 year. Pts who switched from placebo to UPA at wk 14 showed a similar efficacy response compared with those who received continuous UPA. No new safety findings were observed compared with safety data from the UPA clinical development program in other indications.2
- van der Heijde D, et al. Lancet. 2019;394(10214):2108-2117.
- Cohen, et al. Arthritis Rheumatol. 2019;71(suppl 10).
 Figure. Efficacy Endpoints Over Time
Figure. Efficacy Endpoints Over Time
To cite this abstract in AMA style:
Deodhar A, van der Heijde D, Sieper J, Van den Bosch F, Maksymowych W, Kim T, Kishimoto M, Östör A, Combe B, Sui Y, Wang X, Chu A, Song I. Efficacy and Safety of Upadacitinib in Patients with Active Ankylosing Spondylitis: 1-Year Results from a Randomized, Double-Blind, Placebo-Controlled Study with Open-Label Extension [abstract]. Arthritis Rheumatol. 2020; 72 (suppl 10). https://acrabstracts.org/abstract/efficacy-and-safety-of-upadacitinib-in-patients-with-active-ankylosing-spondylitis-1-year-results-from-a-randomized-double-blind-placebo-controlled-study-with-open-label-extension/. Accessed .« Back to ACR Convergence 2020
ACR Meeting Abstracts - https://acrabstracts.org/abstract/efficacy-and-safety-of-upadacitinib-in-patients-with-active-ankylosing-spondylitis-1-year-results-from-a-randomized-double-blind-placebo-controlled-study-with-open-label-extension/