Session Information
Session Type: Poster Session D
Session Time: 9:00AM-11:00AM
Background/Purpose: Tanezumab is a monoclonal antibody against nerve growth factor being investigated for treatment of the signs and symptoms of osteoarthritis (OA). Subcutaneous tanezumab was efficacious, based on Western Ontario and McMaster Universities Osteoarthritis Index (WOMAC) Pain, WOMAC Function, and Patient’s Global Assessment of Osteoarthritis scores, in 2 recent Phase 3 studies, one in North America, and one in Europe and Japan in patients with moderate-to-severe OA of the knee or hip for whom standard of care treatment was not adequate. The current pooled analysis assessed the onset and maintenance of analgesic response to tanezumab in these patients, based on daily pain scores recorded in an electronic diary (e-diary).
Methods: In study A4091056 (clinicaltrials.gov: NCT02697773), patients received 16-week treatment with placebo, tanezumab 2.5 mg (baseline and week 8), or tanezumab 2.5/5 mg (2.5 mg at baseline and 5 mg at week 8; included in the 5 mg group for the current pooled 16-week analysis). In study A4091057 (clinicaltrials.gov: NCT02709486), patients received placebo or tanezumab (2.5 mg or 5 mg) every 8 weeks for 24 weeks. Average pain over the past 24 hours was collected daily in the e-diary during the treatment period using an 11-point numeric rating scale (NRS; from 0 = no pain to 10 = worst possible pain). In the current analysis, the proportion of patients who achieved ≥30% or ≥50% decrease from baseline in NRS pain scores, based on weekly average of daily scores, were compared between placebo and tanezumab groups at each study week through week 16 using a logistic regression model. The median time to achieve ≥30% or ≥50% improvements in pain were compared between groups using a Kaplan-Meier analysis.
Results: Overall, 1545 patients were included in the analysis: placebo (n = 514), tanezumab 2.5 mg (n = 514), or tanezumab 5 mg (n = 517). Significant improvement over placebo was observed by Week 1 in both tanezumab groups for the ≥30% level. For the ≥50% level, significant improvement over placebo was observed by Week 1 for tanezumab 2.5 mg and by Week 2 for tanezumab 5 mg. These significant differences from placebo were maintained at all weeks up to Week 16 for both tanezumab groups at both response levels (p< 0.05 Figures 1 and 2). The time to achieve 30% and 50% response was significantly faster in the tanezumab groups than in the placebo group. The median (95% CI) time to onset of a ≥30% decrease in pain score from baseline was approximately 3 weeks for the tanezumab 2.5 mg group (range 3-4 weeks; p< 0.0001) and 4 weeks for 5 mg (range 3-5 weeks; p=0.0002) compared to 8 weeks for placebo (range 6-10 weeks; Table 1). The median time to onset of a ≥50% decrease in pain score from baseline was approximately 11 weeks in the tanezumab 2.5 mg and 5 mg groups (ranges 9-15 and 9-13 weeks, respectively; p< 0.0001). Not enough patients achieved a 50% response in the placebo group to calculate median time to onset (Table 1).
Conclusion: Tanezumab treatment resulted in significantly more patients reaching moderate (30%) or substantial (50%) improvements in pain compared with placebo as early as Week 1 and through Week 16. The median time to reach ≥30% and ≥50% improvement were 3-4 weeks and 11 weeks, respectively for tanezumab treated patients.
 Figure 1. Proportion of patients with a ≥ 30% improvement in pain score
Figure 1. Proportion of patients with a ≥ 30% improvement in pain score
 Figure 2. Proportion of patients with a ≥ 50% improvement in pain score
Figure 2. Proportion of patients with a ≥ 50% improvement in pain score
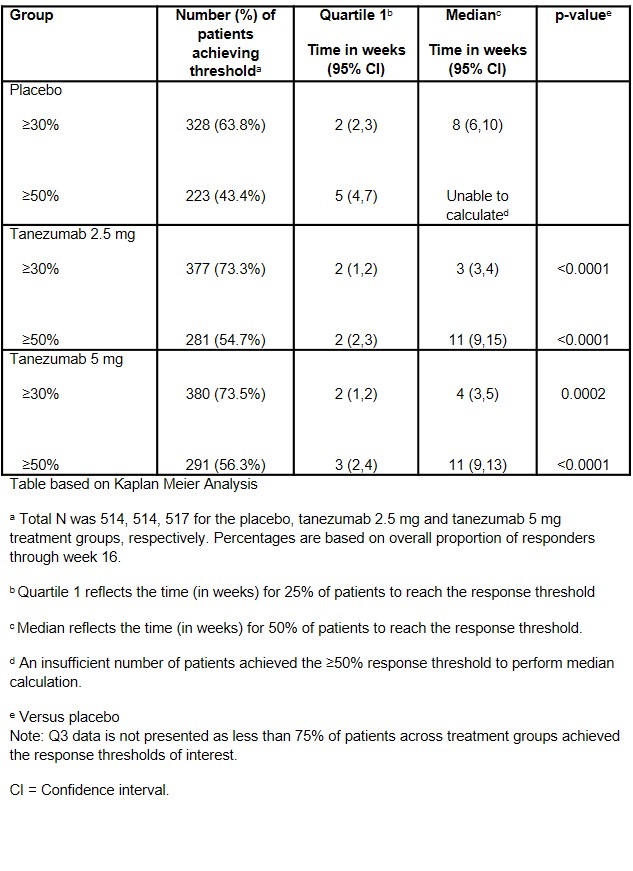 Table 1. Time to ≥30% and ≥50% Improvement from Baseline in Pain Numeric Scale score
Table 1. Time to ≥30% and ≥50% Improvement from Baseline in Pain Numeric Scale score
To cite this abstract in AMA style:
Schnitzer T, Berenbaum F, Davignon I, Yang R, Viktrup L, West C, Verburg K. Evaluating Analgesic Response to Subcutaneous Tanezumab in Patients with Inadequate Treatment Response to Other Analgesics Based on Daily E-pain Diaries: A Pooled Analysis of 2 Randomized, Placebo-controlled Studies [abstract]. Arthritis Rheumatol. 2020; 72 (suppl 10). https://acrabstracts.org/abstract/evaluating-analgesic-response-to-subcutaneous-tanezumab-in-patients-with-inadequate-treatment-response-to-other-analgesics-based-on-daily-e-pain-diaries-a-pooled-analysis-of-2-randomized-placebo-con/. Accessed .« Back to ACR Convergence 2020
ACR Meeting Abstracts - https://acrabstracts.org/abstract/evaluating-analgesic-response-to-subcutaneous-tanezumab-in-patients-with-inadequate-treatment-response-to-other-analgesics-based-on-daily-e-pain-diaries-a-pooled-analysis-of-2-randomized-placebo-con/
