Session Information
Date: Monday, November 9, 2020
Title: Miscellaneous Rheumatic & Inflammatory Diseases Poster III: Therapies
Session Type: Poster Session D
Session Time: 9:00AM-11:00AM
Background/Purpose: Autoinflammatory periodic fever syndromes characterized by excessive interleukin(IL)-1ß release and severe systemic and organ inflammation have been successfully treated with the anti-IL-1ß inhibitor canakinumab. In clinical trial situations and real life, rapid remission of symptoms and normalization of laboratory parameters were observed in most patients.1-3
The present study explores long-term effectiveness and safety of canakinumab under routine clinical practice conditions in pediatric and adult patients with CAPS (cryopyrin-associated periodic syndromes), FMF (familial Mediterranean fever), TRAPS (tumor necrosis factor receptor-associated periodic syndrome) and HIDS/MKD (hyperimmunoglobulinemia D syndrome/mevalonate kinase deficiency).
1Lachmann et al. N Engl J Med. 2009;360(23):2416-25
2Kuemmerle-Deschner et al. Rheumatology (Oxford). 2016;55(4):689-96
3De Benedetti et al. N Engl J Med. 2018;378(20):1908-1919
Methods: RELIANCE is a prospective, non-interventional, multi-center, observational study based in Germany with a 3-year follow-up period. Pediatric (age ≥2 years) and adult patients with clinically confirmed diagnoses of CAPS, FMF, TRAPS and HIDS/MKD that routinely receive canakinumab are enrolled in order to evaluate effectiveness and safety of canakinumab under standard clinical practice conditions. Evaluation of disease activity and fatigue by patients’ assessment, days absent from school/work due to study indication, inflammatory markers and physician assessment of disease remission was performed at baseline and will be further assessed at 6-monthly intervals within the 3-year observation period of the study.
Results: This first interim analysis of 41 patients enrolled by September 2019, diagnosed with FMF (n=29), TRAPS (n=10) and HIDS/MKD (n=2), includes baseline data as well as preliminary 12-month data of a FMF subset (N=16). Mean age at baseline for FMF-TRAPS-HIDS/MKD patients was 26-22-11 years and mean duration of prior canakinumab treatment was 2.2-1-3 years.
Preliminary results of the FMF subset of N=16 patients diagnosed with FMF indicate stable remission and disease control during observation period. Within the first study interval, no major changes were observed regarding physician ratings, patient reported outcomes and inflammatory markers (Table 1). The AIDAI score of disease activity revealed inactive disease in 9 out of 12 months (Table 2). Preferred canakinumab doses and intervals (% of all injections) were 150 mg every 4 weeks (24), 150 mg every 6 weeks (11), and 150 mg every 8 weeks (21). Any serious adverse events were reported for 2 patients including tonsillectomy (drug-related) and arthritis. No study discontinuations were observed.
Conclusion: Baseline characteristics of the FMF-TRAPS-HIDS/MKD-subgroup are reported. In addition, first interim data of FMF patients available from the RELIANCE registry show that long-term CAN treatment is well tolerated and effective in FMF patients.
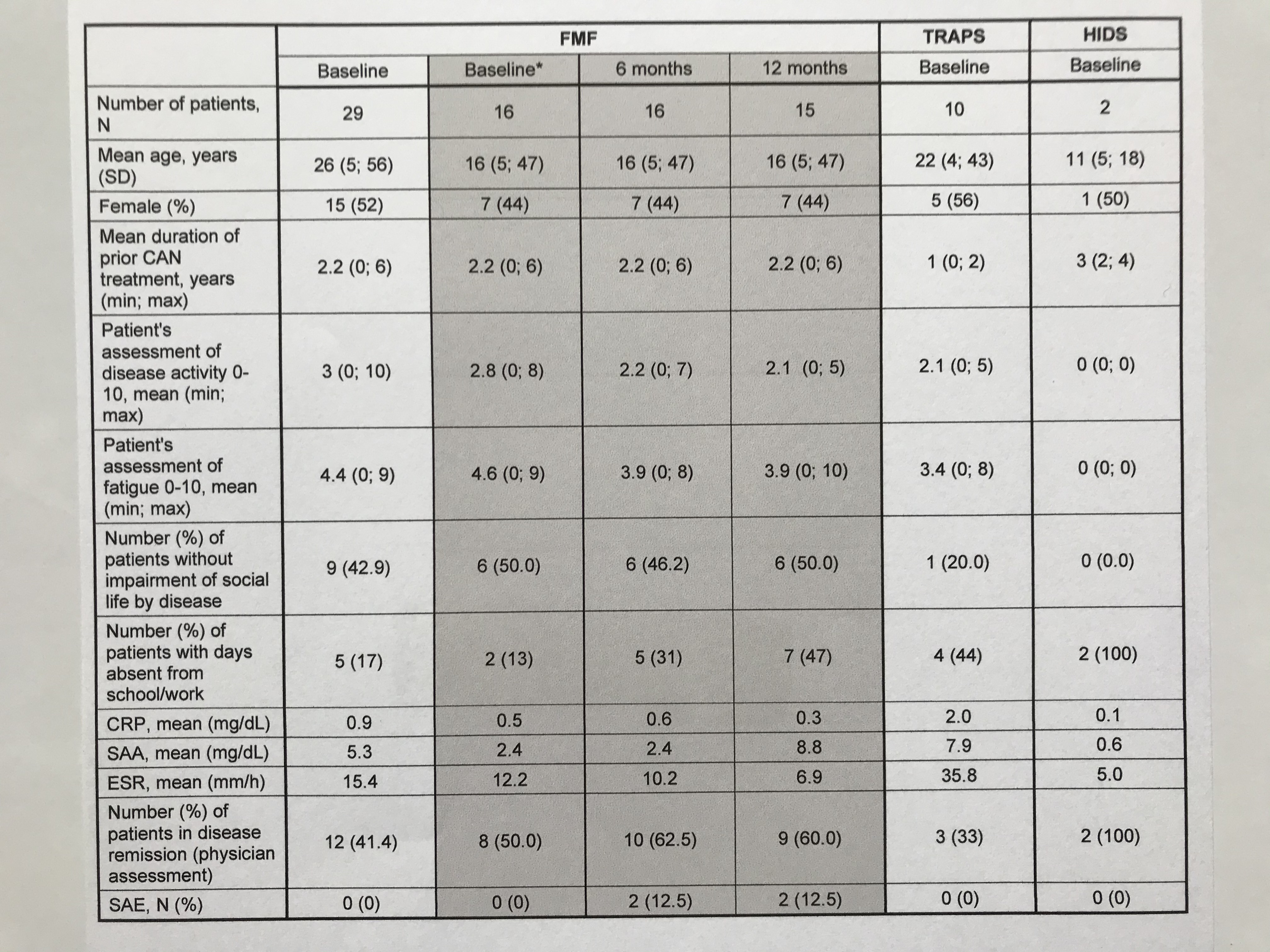 Table 1: Baseline characteristics of the FMF/TRAPS/HIDS-subgroup and preliminary 6-month interim data of 16 FMF patients *Baseline of FMF patient subset (N=16) with preliminary 6 and 12-month data
Table 1: Baseline characteristics of the FMF/TRAPS/HIDS-subgroup and preliminary 6-month interim data of 16 FMF patients *Baseline of FMF patient subset (N=16) with preliminary 6 and 12-month data
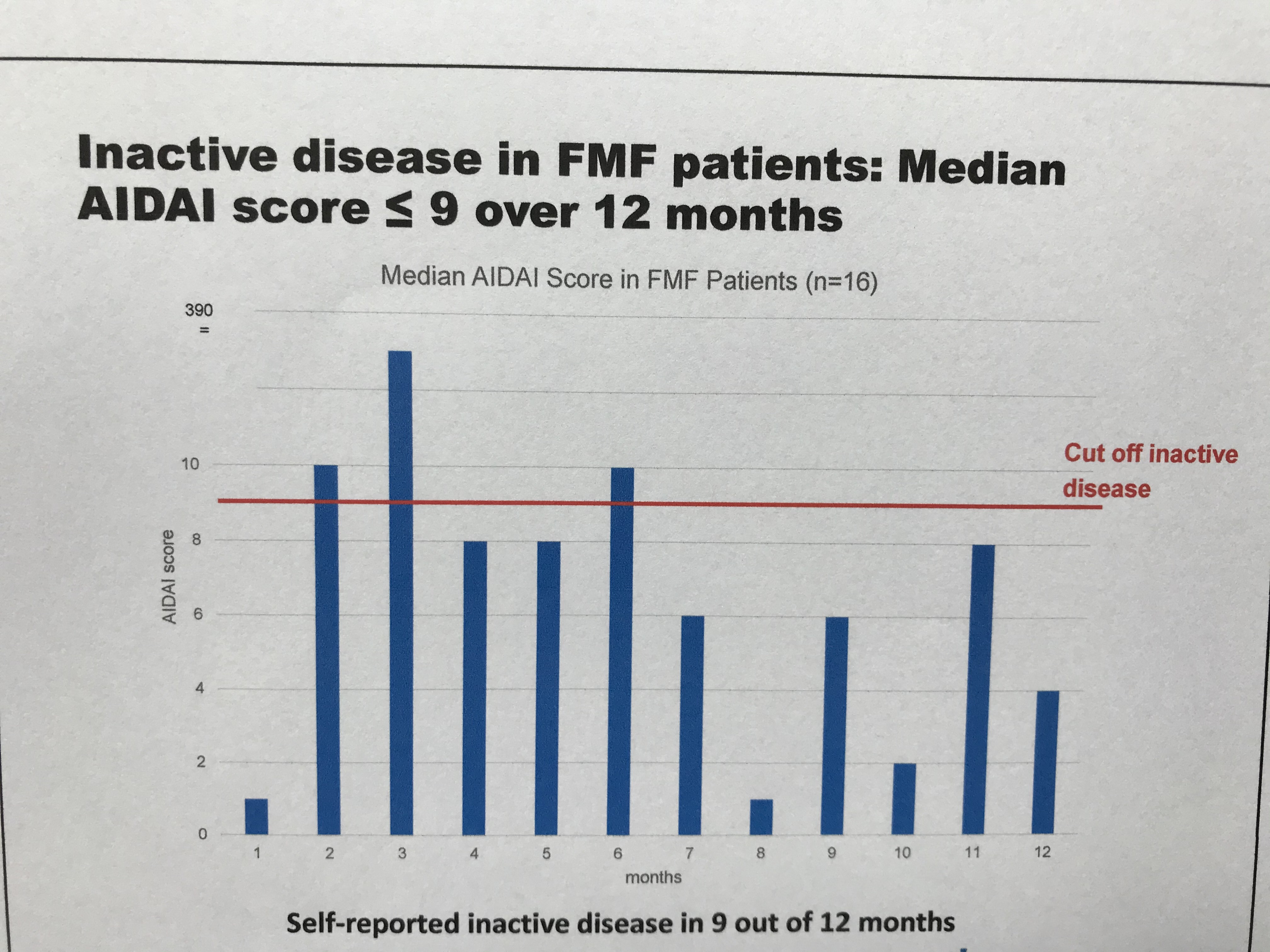 Table 2: Median AIDAI Score of the FMF subgroup (n=16) Over 12 Months
Table 2: Median AIDAI Score of the FMF subgroup (n=16) Over 12 Months
To cite this abstract in AMA style:
Henes J, Blank N, Borte M, Foeldvari I, Horneff G, Hufnagel M, Kallinich T, Kortus-Goetze B, Schuetz C, Weller-Heinemann F, Weber-Arden J, Kuemmerle-Deschner J. Long-Term Efficacy and Safety of Canakinumab in Patients with Autoinflammatory Periodic Fever Syndromes – First Interim Analysis of the FMF-TRAPS-HIDS/MKD Subgroup of the RELIANCE Registry [abstract]. Arthritis Rheumatol. 2020; 72 (suppl 10). https://acrabstracts.org/abstract/long-term-efficacy-and-safety-of-canakinumab-in-patients-with-autoinflammatory-periodic-fever-syndromes-first-interim-analysis-of-the-fmf-traps-hids-mkd-subgroup-of-the-reliance-registry/. Accessed .« Back to ACR Convergence 2020
ACR Meeting Abstracts - https://acrabstracts.org/abstract/long-term-efficacy-and-safety-of-canakinumab-in-patients-with-autoinflammatory-periodic-fever-syndromes-first-interim-analysis-of-the-fmf-traps-hids-mkd-subgroup-of-the-reliance-registry/
