Session Information
Session Type: Poster Session D
Session Time: 9:00AM-11:00AM
Background/Purpose: Chronic glucocorticoid treatment in children increases the risk of bone loss, fractures, and reduced adult skeletal mass. The 2017 ACR Glucocorticoid-Induced Osteoporosis (GIOP) guidelines recommend optimizing vitamin D intake to mitigate these risks. We performed a quality improvement project to measure and improve provider adherence to this guideline in an academic pediatric rheumatology clinic.
Methods: This project was led by pediatric and adult rheumatology fellows at our institution as part of our annual quality improvement curriculum. Our primary aim was to increase the percentage of pediatric patients treated with glucocorticoids prescribed a vitamin D supplement from less than 30% to 50%. A secondary measure was the percentage of patients on glucocorticoids with a vitamin D level checked in the preceding year. Using Plan-Do-Study-Act (PDSA) methodology, we conducted these cycles: (1) a combined pediatric and adult rheumatology divisional grand rounds describing the project, (2) a pediatric rheumatology conference focused on discussion of the project, and (3) a presentation on bone health by a pediatric endocrinologist. Data were obtained using Slicer Dicer, an analytic data tool available within our electronic health record (EPIC). Data were validated at baseline by manual chart review of all patients taking glucocorticoids in pediatric rheumatology clinic in October 2019. Additional analyses were performed based on rheumatologic diagnosis (defined by ICD-10 codes) and age. Data were analyzed using descriptive statistics.
Results: The Slicer Dicer search at baseline in July 2019 revealed that 21.4% of patients on glucocorticoids were prescribed a vitamin D supplement, and 28.6% had a vitamin D level checked in the preceding year. By March 2020, vitamin D supplementation increased to 54.5% (Figure 1), and the percentage of patients with a vitamin D level checked increased to 50%. The largest rise in vitamin D prescriptions occurred following PDSA cycle 3.
Throughout the project, vitamin D supplementation in patients with SLE was 70.6% (n=34), other connective tissue diseases 30.4% (n=23), vasculitis 28.6% (n=7), and JIA 11.5% (n=26) (Figure 2). When stratifying by age, vitamin D supplementation was 10% in patients < 5 (n=10), 7.5% in patients 5 to 13 (n=40), and 43.4% in patients ≥13 (n=83) (Figure 3). Rates of vitamin D levels checked were similar to vitamin D supplementation rates in each group.
Manual review of 31 patient charts in October 2019 revealed that 69% were prescribed glucocorticoids for >3 months. Of the patients prescribed chronic glucocorticoids, 45% had an active vitamin D prescription, and 37.5% had a preceding vitamin D level. Analysis by Slicer Dicer found that 45% were prescribed vitamin D and 35.5% had a preceding vitamin D level in October 2019.
Conclusion: We met our aim of increasing the percentage pediatric rheumatology patients on glucocorticoids taking vitamin D supplementation from 30% to 50%. The largest rise occurred after education from pediatric endocrinology. The highest levels of vitamin D supplementation were seen in patients with SLE and patients aged 13 to 25. Future interventions could focus on other diagnoses and age groups.
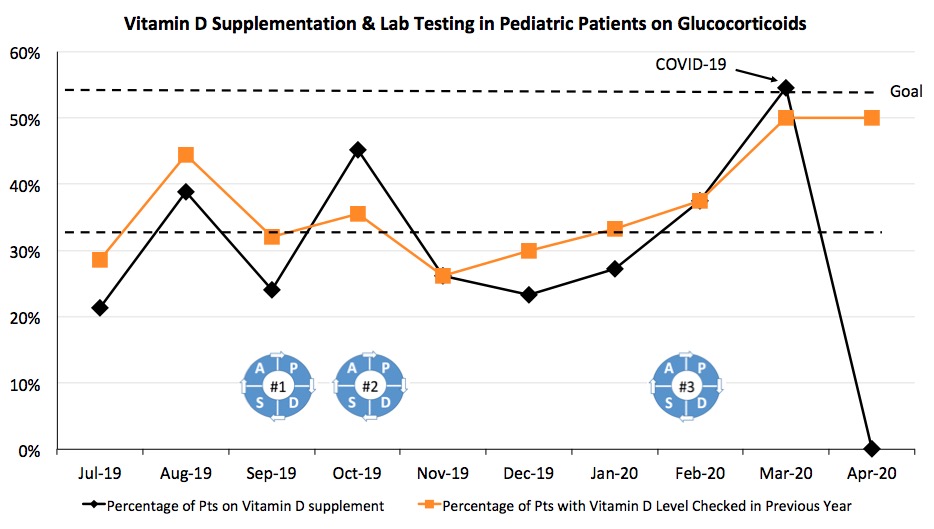 Figure 1. Run chart for vitamin D supplementation (black) and vitamin D levels checked (orange) by month in pediatric patients on glucocorticoids. The PDSA cycles are as follows: 9/17/2019- Combined Adult and Pediatric Rheumatology QI Introductory Grand Rounds; 10/3/2019- Pediatric Divisional QI Introduction; 2/6/2020- Pediatric Endocrinology Bone Health Educational Lecture. Our academic center started seeing an impact from the COVID-19 infection in mid-March 2020.
Figure 1. Run chart for vitamin D supplementation (black) and vitamin D levels checked (orange) by month in pediatric patients on glucocorticoids. The PDSA cycles are as follows: 9/17/2019- Combined Adult and Pediatric Rheumatology QI Introductory Grand Rounds; 10/3/2019- Pediatric Divisional QI Introduction; 2/6/2020- Pediatric Endocrinology Bone Health Educational Lecture. Our academic center started seeing an impact from the COVID-19 infection in mid-March 2020.
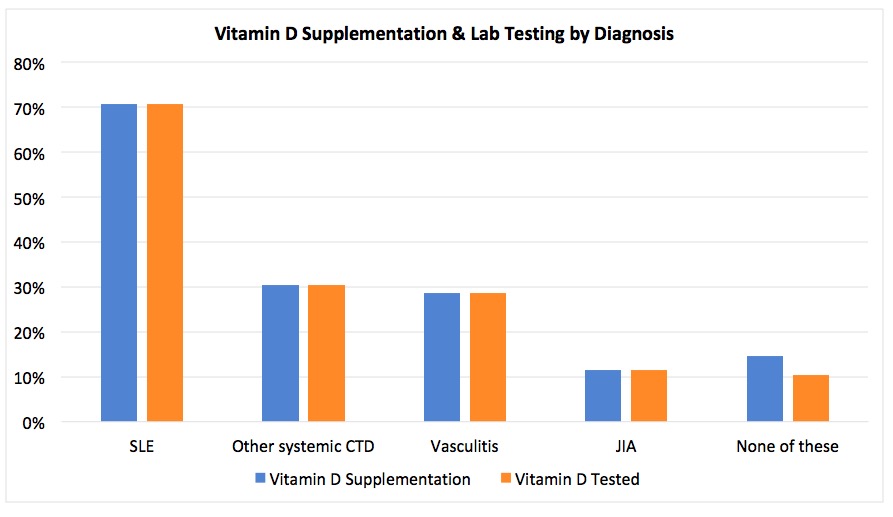 Figure 2. The percentage of patients on glucocorticoids who have an active prescription for a vitamin D supplement (blue) and who have had a vitamin D level checked in the preceding year (orange) by diagnosis. Other CTDs = JDM, localized scleroderma, SS, SSc, UCTD.
Figure 2. The percentage of patients on glucocorticoids who have an active prescription for a vitamin D supplement (blue) and who have had a vitamin D level checked in the preceding year (orange) by diagnosis. Other CTDs = JDM, localized scleroderma, SS, SSc, UCTD.
 Figure 3. The percentage of patients on glucocorticoids who have an active prescription for a vitamin D supplement (blue) and who have had a vitamin D level checked in the preceding year (orange) by age. Other CTDs = JDM, localized scleroderma, SS, SSc, UCTD.
Figure 3. The percentage of patients on glucocorticoids who have an active prescription for a vitamin D supplement (blue) and who have had a vitamin D level checked in the preceding year (orange) by age. Other CTDs = JDM, localized scleroderma, SS, SSc, UCTD.
To cite this abstract in AMA style:
Kaufman K, Buckley M, Cannon L, Randell R, Chu P, Maheswaranathan M, Johannemann A, Anderson D, Smith I, Udupa A, Leverenz D. A Quality Improvement Project to Increase Vitamin D Prescribing for Pediatric Patients on Glucocorticoids [abstract]. Arthritis Rheumatol. 2020; 72 (suppl 10). https://acrabstracts.org/abstract/a-quality-improvement-project-to-increase-vitamin-d-prescribing-for-pediatric-patients-on-glucocorticoids/. Accessed .« Back to ACR Convergence 2020
ACR Meeting Abstracts - https://acrabstracts.org/abstract/a-quality-improvement-project-to-increase-vitamin-d-prescribing-for-pediatric-patients-on-glucocorticoids/
