Session Information
Date: Sunday, November 8, 2020
Title: SLE – Diagnosis, Manifestations, & Outcomes III: Lupus Nephritis (1512–1516)
Session Type: Abstract Session
Session Time: 4:00PM-4:50PM
Background/Purpose: Lupus nephritis continues to be the complication with the highest standardized mortality ratio in Systemic Lupus Erythematosus (SLE), and a late diagnosis associates with worse outcomes. Clinicians traditionally rely on proteinuria to drive decisions regarding renal biopsy and subsequent management. Since threshold levels for such determinations are variable but critically important, this study leveraged the well-phenotyped multi-center multi- racial Accelerating Medicines Partnership (AMP) lupus nephritis cohort, to address whether urine protein to creatinine ratios (UPCR) between .5 and 1 differ from higher ratios with regard to clinical, serologic and histologic variables.
Methods: 239 patients fulfilling ACR or SLICC criteria for SLE with a random or 24 hr uPCR > or = .5 and histologic biopsy Class III, IV, V, or mixed were consecutively enrolled in AMP at the time of renal biopsy and demographics, clinical history, medications, disease activity as assessed by the hybrid SELENA-SLEDAI were recorded. Patients with biopsy Classes I, II and VI were ineligible. Patients were followed at 3, 12, 26 and 52 weeks.
Results: At baseline, 38 patients had a UPCR < 1 (A), 113 had a UPCR 1-3 (B) , and 88 had a UPCR > 3 (C). There were 14 additional patients with UPCR < 1, and 11 patients with UPCR > 1 who had biopsy class I or II. In group A, there were significantly more male patients (44% A; 23% B; 26% C, p=0.012) with no differences in age, race or ethnicity. Neither the SLEDAI nor serologic parameters (anti-dsDNA, C3, or C4) distinguished among the groups. Those in group C had a significantly increased creatinine and decreased hemoglobin and albumin compared to the other two groups (Table 1). Patients in group A trended toward having an increased frequency of proliferative histology (Table 2). This trend was not observed when considering patients for whom this was their first biopsy, but was significant for repeat biopsy patients (56% A; 41% B; 24% C, p=0.03). The activity index was independent of UPCR regardless of biopsy number. However, those in group C had a significantly higher chronicity index than those with lower UPCR. This correlation was shown for patients with a repeat biopsy (r=0.2299, p=0.003) but not first biopsy patients (r=0.0891, p=0.45). Although medications did not differ at baseline among the groups, at 12 weeks, for each group significantly more patients were taking Mycophenolate Mofetil than at the time of biopsy (Table 3).
Conclusion: A significant proportion of both first and recurrent biopsies in patients with a UPCR < 1 have proliferative histology and accompanying activity scores similar to that of patients with nephrotic range proteinuria. These results support renal biopsy at thresholds lower than a UPCR of 1 since histologic findings can inform therapeutic decisions.
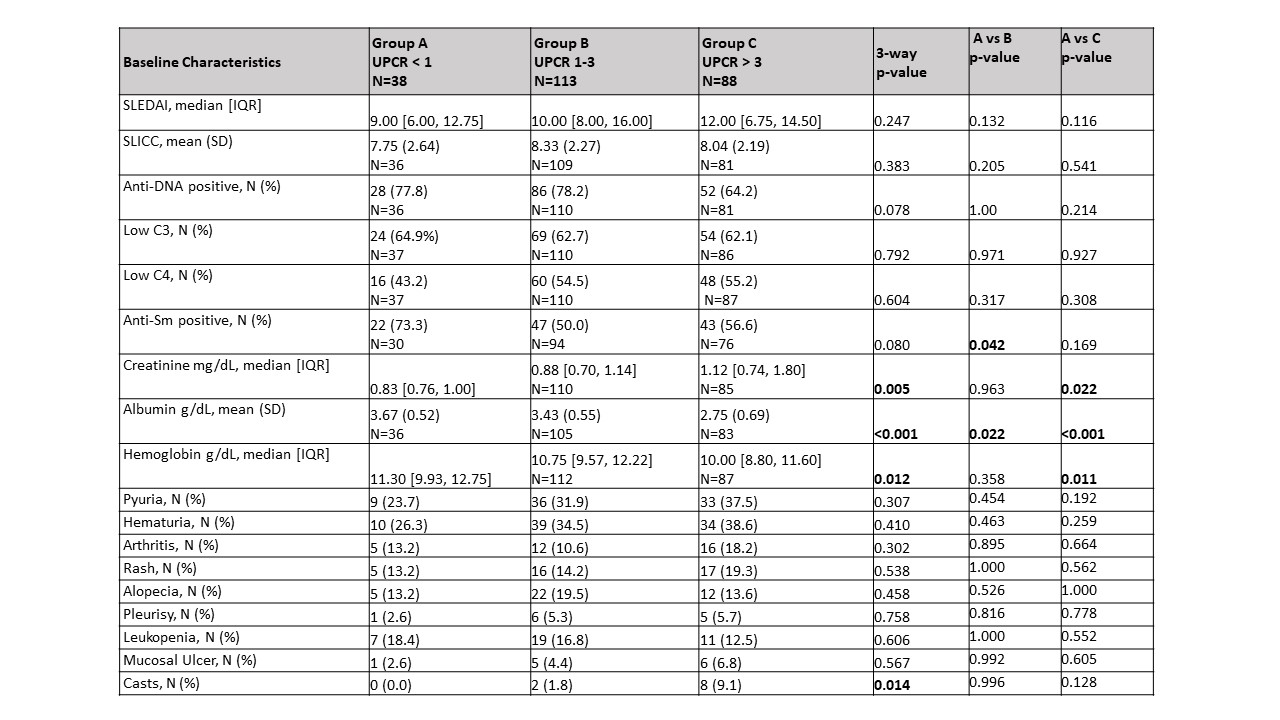 Table 1: Baseline lupus activity by UPCR group.
Table 1: Baseline lupus activity by UPCR group.
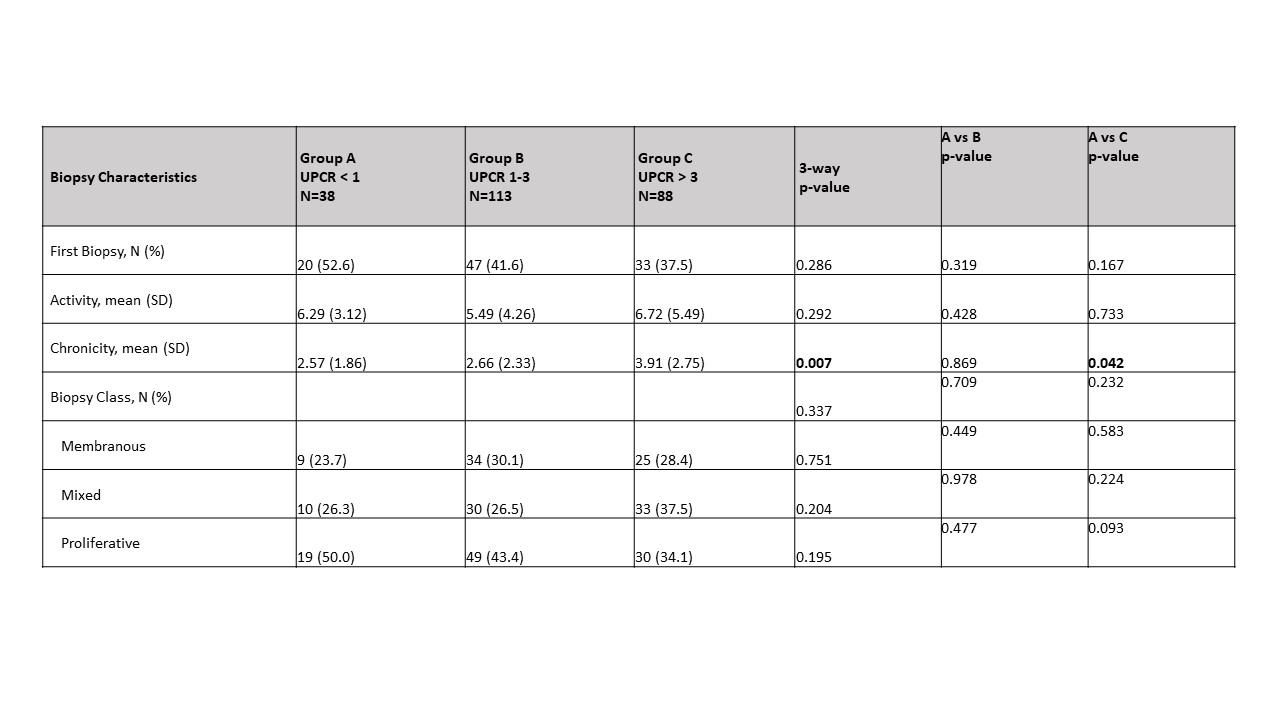 Table 2: Biopsy characteristics by UPCR group.
Table 2: Biopsy characteristics by UPCR group.
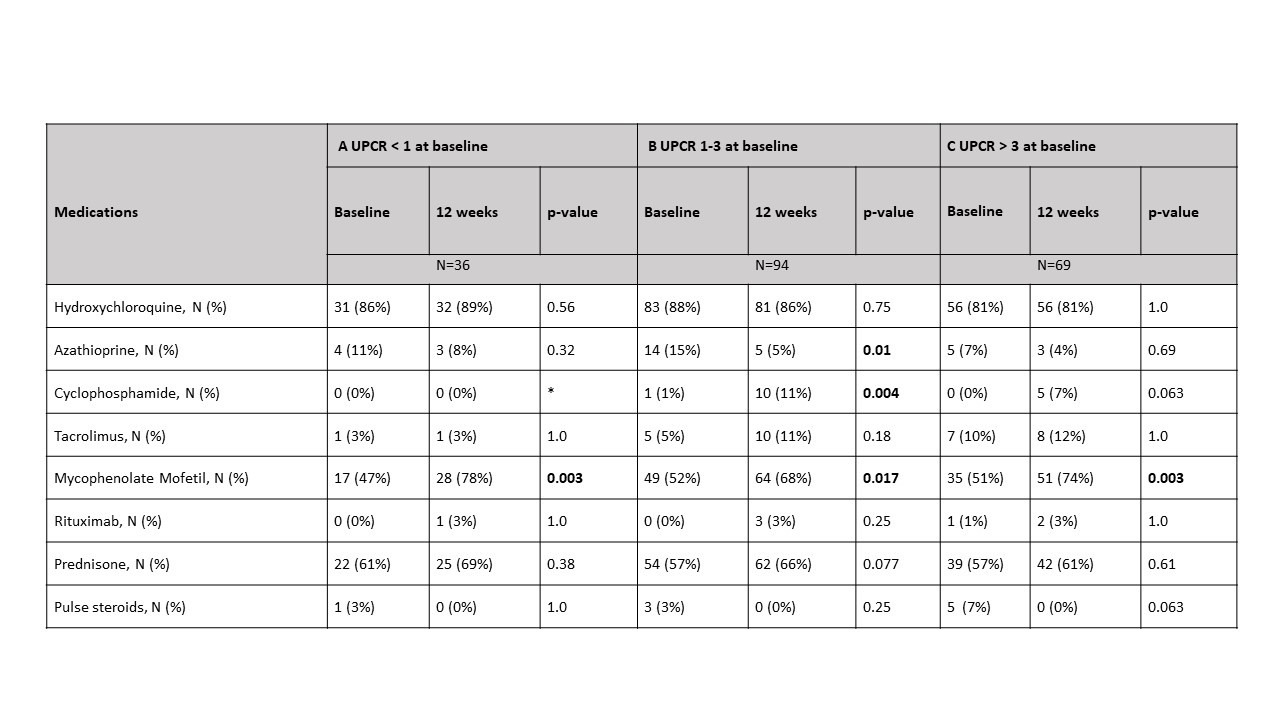 Table 3: Comparison of medications at baseline and 12 weeks for each UPCR group
Table 3: Comparison of medications at baseline and 12 weeks for each UPCR group
To cite this abstract in AMA style:
Carlucci P, Deonaraine K, Fava A, Li J, Wofsy D, James J, Putterman C, Diamond B, Fine D, Monroy-Trujillo J, Haag K, Apruzzese W, Belmont H, Izmirly P, Connery S, Payan-Schober F, Furie R, Berthier C, Dall'Era M, Cho K, Kamen D, Kalunian K, Accelerating Medicines Partnership in SLE Network T, Petri M, Buyon J. The Value of Renal Biopsy at Lower Levels of Proteinuria in Patients Enrolled in the Lupus Accelerating Medicines Partnership [abstract]. Arthritis Rheumatol. 2020; 72 (suppl 10). https://acrabstracts.org/abstract/the-value-of-renal-biopsy-at-lower-levels-of-proteinuria-in-patients-enrolled-in-the-lupus-accelerating-medicines-partnership/. Accessed .« Back to ACR Convergence 2020
ACR Meeting Abstracts - https://acrabstracts.org/abstract/the-value-of-renal-biopsy-at-lower-levels-of-proteinuria-in-patients-enrolled-in-the-lupus-accelerating-medicines-partnership/
