Session Information
Session Type: Poster Session C
Session Time: 9:00AM-11:00AM
Background/Purpose: Tocilizumab (TCZ) has been proven to be safe and effective for the treatment of giant cell arteritis (GCA) in 2 randomized controlled trials; however, data from additional types of studies provide valuable information related to the treatment of GCA with TCZ. The objective of this study was to review and analyze efficacy and safety data for TCZ in GCA based on peer-reviewed publications to date.
Methods: A systematic literature review was conducted according to the PRISMA guidelines. Publications were retrieved from the MEDLINE, Embase, Cochrane, Scopus and Web of Science databases. Publications of clinical trials and retrospective or prospective observational studies (April 11, 2005-October 8, 2019) including patients with GCA (classified based on ACR criteria and/or positive biopsy vs imaging) treated with TCZ and reporting a measure of efficacy were eligible for inclusion. Extracted data included year of publication, year(s) when the study was conducted, number of patients with GCA, method of GCA diagnosis, age and sex, TCZ treatment details (dose, route of administration, frequency, duration), clinical outcome (remission, relapse), serious adverse events (SAEs) following treatment with TCZ, corticosteroid dose before and following TCZ initiation and imaging data following TCZ treatment. Results are presented for the studies (unweighted) and as the weighted population mean. Unweighted mean proportions were calculated as the average of the proportions reported from each study; unweighted means were calculated by computing the mean of individual reported study means. The weighted population proportion was estimated by calculating the total number of patients achieving an outcome and dividing by the total number of patients in all of the studies. The weighted population mean was equivalent to the sum of the individual patient’s outcome values divided by the total number of patients in all of the studies.
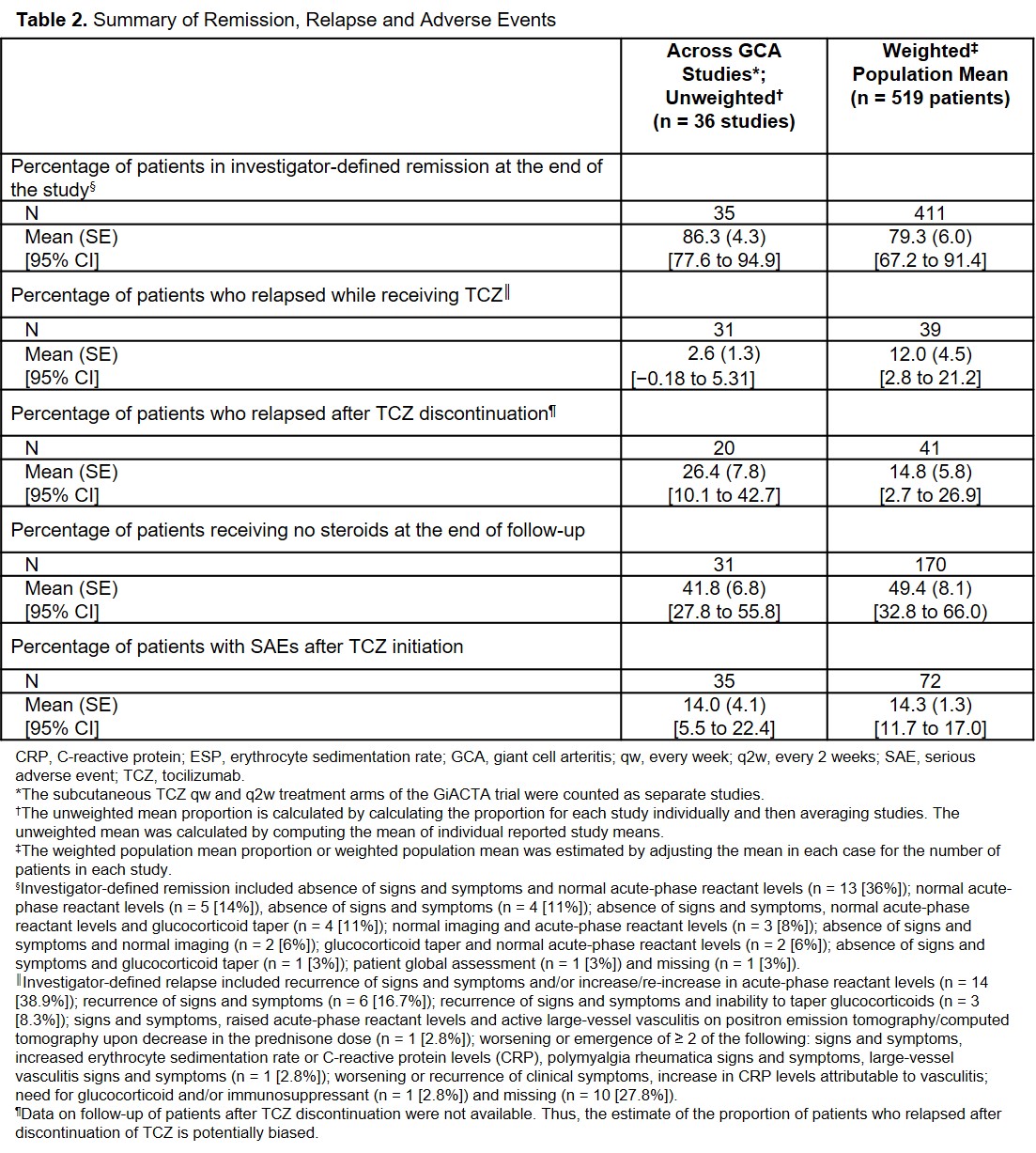
Results: The search retrieved 664 references; 55 full-text articles were reviewed for eligibility, and 36 studies were included in the meta-analysis (Figure 1); the once-weekly and every-2-weeks subcutaneous (SC) TCZ arms of the GiACTA trial were counted as separate studies. A total of 519 patients were included. The median (IQR) duration of treatment with intravenous and SC TCZ was 26.0 (20.0-37.3) and 52.0 (26.0-52.0) weeks, respectively, across all studies in the unweighted analysis and 25.8 (20.8-41.9) and 38.9 (32.4-45.5) weeks in the weighted analysis (Table 1). The mean (SE) proportion of patients achieving investigator-defined remission at the end of the study was 86.3% (4.3%) and 79.3% (6.0%) in the unweighted and weighted analyses, respectively (Table 2). The mean (SE) proportion of patients who relapsed while receiving TCZ was 2.6% (1.3%) and 12.0% (4.5%) in the unweighted and weighted analyses, respectively. The median prednisone dose at the end of the study was < 5 mg/day, and the mean proportion of patients with SAEs after TCZ initiation was ≈ 14.0%.
Conclusion: A high proportion of patients with GCA treated with TCZ were in investigator-defined remission at the end of the study across all studies analyzed. These meta-analysis findings add to the evidence of the efficacy and safety of TCZ in GCA.
To cite this abstract in AMA style:
Koster M, Warrington K, Han J, Mohan S. The Efficacy and Safety of Tocilizumab in Patients with Giant Cell Arteritis: A Systematic Review and Meta-Analysis [abstract]. Arthritis Rheumatol. 2020; 72 (suppl 10). https://acrabstracts.org/abstract/the-efficacy-and-safety-of-tocilizumab-in-patients-with-giant-cell-arteritis-a-systematic-review-and-meta-analysis/. Accessed .« Back to ACR Convergence 2020
ACR Meeting Abstracts - https://acrabstracts.org/abstract/the-efficacy-and-safety-of-tocilizumab-in-patients-with-giant-cell-arteritis-a-systematic-review-and-meta-analysis/